| CPC A61K 8/494 (2013.01) [A61K 8/22 (2013.01); A61K 8/24 (2013.01); A61K 8/415 (2013.01); A61Q 5/08 (2013.01); A61Q 5/10 (2013.01); A61K 2800/884 (2013.01)] | 20 Claims |
|
1. A method for dyeing hair, comprising:
(1) treating hair with an oxidative colorant agent (A);
(2) subsequent to (1), treating the hair with a CH-acidic hair dyeing agent (B),
wherein the CH-acidic hair dyeing agent (B) has a pH of from about 7 to about 10, measured at 22° C., and comprises the product of mixing together three agents (B1), (B2), and (B3), where
agent (B1) comprises at least one reactive carbonyl compound in a cosmetic carrier and is free from CH-acidic compounds,
agent (B2) comprises at least one CH-acidic compound in a cosmetic carrier and is free from reactive carbonyl compounds, the at least one CH-acidic compound being selected from compounds of the formula (CH-1) and/or compounds of the formula (CH-2):
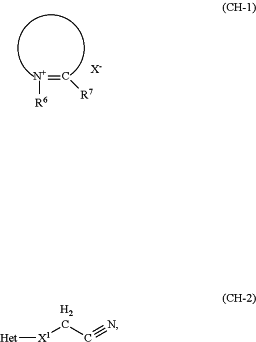 wherein in formula (CH-1):
R6 represents a linear or cyclic (C1 to C6)alkyl group; a (C2 to C6)alkenyl group; an optionally substituted aryl group; an optionally substituted heteroaryl group; an aryl (C1 to C6)alkyl group; a (C1 to C6)hydroxyalkyl group; a (C2 to C6)polyhydroxyalkyl group; a (C1 to C6)alkoxy (C1 to C6)alkyl group; a group of the formula RIRIIN—(CH2)m—, in which m is an integer of from 2 to 6, and RI and RII each independently of one another represent a hydrogen atom, a (C1 to C4)alkyl group, a (C1 to C4)hydroxyalkyl group, or an aryl-(C1 to C6)alkyl group, it being where RI and RII together with the nitrogen atom N optionally form a 5-, 6-, or 7-membered ring,
R7 represents a (C1 to C6)alkyl group, and
X−is a physiologically compatible anion, and
where the formula (CH-1) represents a heterocyclic structure optionally comprising nitrogen, oxygen, and/-or sulfur heteroatoms in a ring structure between N-R6 and C-R7-, where the ring structure may be fused to another ring structure, and where each ring structure in the compound of the formula (CH-1) is optionally substituted with a pendant substituent; and
wherein in (CH-2):
Het represents an optionally substituted heteroaromatic group, and
X1 represents a covalent bond or a carbonyl group (C═O); and
agent (B3) comprises at least one alkalizing agent; and
(3) subsequent to (2), treating the hair with a cosmetic decolorizing agent (Ox),
wherein the cosmetic decolorizing agent (Ox) has a pH value in the range from 4 to 11, measured at 22° C., and comprises water and from about 0.5 to about 12 wt. % hydrogen peroxide, based on the total weight of the decolorizing agent (Ox);
wherein the method comprises a time interval between (1) and (2) of from about 1 minute to about 1.5 months, and a time interval between (2) and (3) of from about 1 minute to about 1.5 months.
|