| CPC A61K 47/6885 (2017.08) [A61K 9/0019 (2013.01); A61K 45/06 (2013.01); A61K 47/60 (2017.08); C08G 83/003 (2013.01); C08G 83/004 (2013.01); A61K 47/183 (2013.01)] | 28 Claims |
|
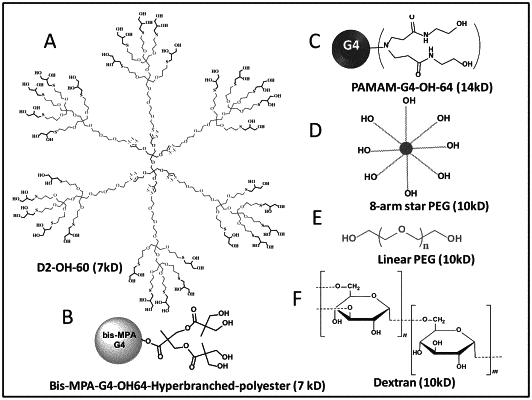
1. A dendrimer comprising a central core, one or more branching units,
and a plurality of terminal hydroxyl groups,
wherein the dendrimer is a generation 2 dendrimer having a surface density of the hydroxyl groups of at least three hydroxyl groups/nm2, as measured by dynamic light scattering,
wherein the central core is prepared from one or more compounds selected from the group consisting of dipentaerythritol, azide-modified dipentaerythritol, alkyne-modified dipentaerythritol, pentaerythritol, azide-modified pentaerythritol, and alkyne-modified pentaerythritol,
wherein the one or more branching units comprise a linear or branched polyethylene glycol,
wherein one or more prophylactic, therapeutic, or diagnostic agents are encapsulated in, associated with, or conjugated to the dendrimer, and the dendrimer is formulated for systemic administration for local treatment of inflammation mediated by activated macrophages or microglia.
|