| CPC A61K 31/675 (2013.01) [A61K 31/506 (2013.01); A61K 31/5377 (2013.01); A61K 45/06 (2013.01); A61P 35/00 (2018.01); C07D 403/04 (2013.01); C07D 403/14 (2013.01); C07D 413/14 (2013.01); C07D 471/04 (2013.01); C07D 487/08 (2013.01); C07F 9/65583 (2013.01)] | 26 Claims |
|
1. A compound of Formula I:
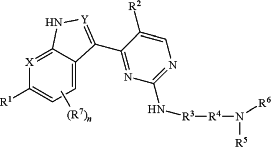 or a pharmaceutically acceptable salt, stereoisomer, or isotopic form thereof, wherein:
X is N, CH, or C(R10), wherein R10 is halo or —P(O)(CH3)2;
Y is N or CH;
R1 is —OH, —CN, —C(O)NH2, or one of the following heteroaryl groups
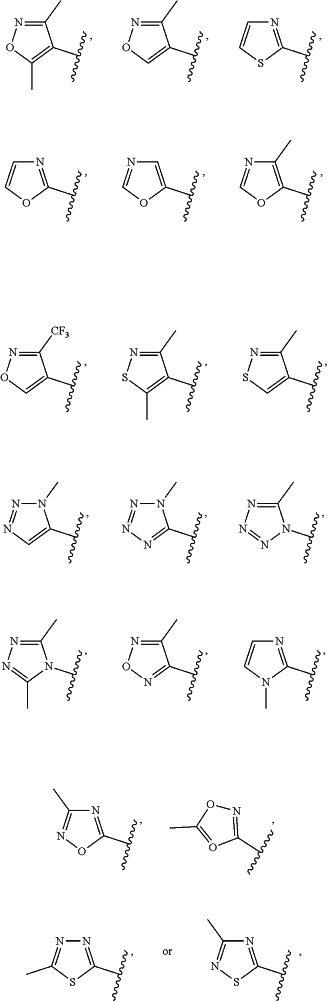 wherein any heteroaryl portion of R1 is optionally substituted at up to two substitutable carbon atoms with a substituent independently selected from halo, —CN, —OH, —NH2, —NH(C1-C4 alkyl), —N(C1-C4 alkyl)2, C1-C4 alkyl, —O—(C1-C4 alkyl), —(C1-C4 alkylene)-O—(C1-C4 alkyl), and —S(O)2—(C1-C4 alkyl);
R2 is fluoro, chloro, —CN, or C1-C4 alkyl optionally substituted with fluoro;
R3, R4, and R5 are as follows:
(i) R3 is C or CH and R4 is C or CH, wherein R3 and R4 are taken together to form an optionally substituted and optionally benzofused cycloalkyl, and R5 is hydrogen, C1-C4 alkyl, or is taken together with R6 to form an optionally substituted and optionally benzofused saturated monocyclic or bicyclic heterocyclyl; or
(ii) R3 is C or CH, R4 is C, CH, or CH2, and R5 is C, CH or CH2, wherein R3 and R5 are taken together with R4 and the intervening nitrogen atom to form a saturated monocyclic heterocyclyl other than piperidine, wherein the saturated monocyclic heterocyclyl is optionally substituted and optionally fused to a cycloalkyl, saturated heterocyclyl, or phenyl ring, wherein R5 and R6 are optionally taken together to form a ring that is fused to the saturated monocyclic heterocyclyl formed when R3 and R5 are taken together; or
(iii) R3 is CH2, R4 is C or CH, and R5 is C, CH or CH2, wherein R4 and R5 are taken together with the intervening nitrogen atom to form an optionally substituted and optionally benzofused saturated monocyclic or bicyclic heterocyclyl;
R6 is hydrogen, —C1-C4 alkyl, —C(O)—(C1-C4 alkyl), —C(O)—(C1-C4 alkylene)-N(R7)2, —(C1-C4 alkylene)-O—(C1-C4 alkyl), —C(O)-aryl, or —S(O)2-aryl;
each R7 is, independently, halo, —CN, —OH, —NH2, —NH(C1-C4 alkyl), N(C1-C4 alkyl)2, —C1-C4 alkyl, —O—(C1-C4 alkyl), —(C1-C4 alkylene)-O—(C1-C4 alkyl) or —S(O)2—(C1-C4 alkyl);
n is 0, 1, 2, 3 or 4;
each saturated heterocyclyl, cycloalkyl, or aryl is optionally substituted with up to four substituents independently selected from halo, —CN, —OH, —NH2, —NH(C1-C4 alkyl), N(C1-C4 alkyl)2, —C1-C4 alkyl, —O—(C1-C4 alkyl), —(C1-C4 alkylene)-O—(C1-C4 alkyl), and an optionally substituted phenyl; and
each C1-C4 alkyl and C1-C4 alkylene is optionally substituted with up to five substituents independently selected from halo, —CN, —OH, —NH2, —NH(unsubstituted C1-C4 alkyl), N(unsubstituted C1-C4 alkyl)2, and —O-(unsubstituted C1-C4 alkyl).
|