| CPC C07K 7/08 (2013.01) [A61K 38/10 (2013.01); A61K 47/55 (2017.08); A61K 47/64 (2017.08); A61K 47/65 (2017.08); A61P 35/00 (2018.01); A61K 31/40 (2013.01); A61K 31/5375 (2013.01); A61K 38/00 (2013.01)] | 18 Claims |
|
1. A method for synthesizing compound BCY8245
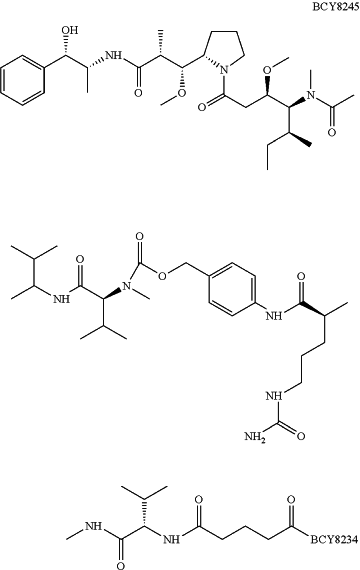 or a salt thereof, the method comprising:
(1) providing a compound having a formula 8
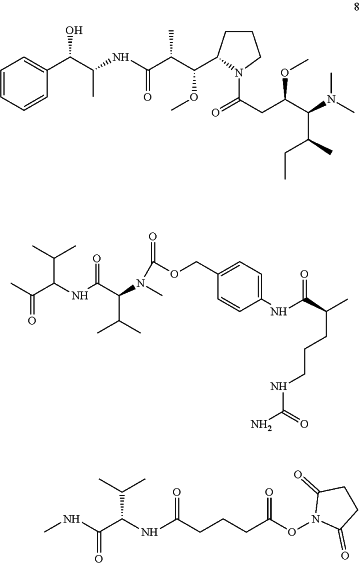 or a salt thereof; and
(2) reacting the compound of formula 8 or the salt thereof with compound BCY8234 or a salt thereof to form compound BCY8245 or the salt thereof;
wherein compound BCY8234 is a bicyclic peptide ligand specific for Nectin-4 comprising a polypeptide and a molecular scaffold, wherein the polypeptide has an amino acid sequence: [β-Ala][Sar10]-(SEQ ID NO: 1);
wherein SEQ ID NO: 1 is CiP[lNal][dD]CiiM[HArg]DWSTP[HyP]WCiii; and
wherein 1NaI represents 1-naphthylalanine, HArg represents homoarginine, HyP represents hydroxyproline, and Ci,Cii, and Ciii represent, respectively, first, second, and third cysteine residues; and
wherein the molecular scaffold is 1,1 ‘,1 “-(1,3,5-tri azinane-1,3, 5-triyl)triprop-2-en-1-one (TATA), which forms covalent bonds with the cysteine residues of the polypeptide such that two polypeptide loops are formed on the molecular scaffold.
|