| CPC C07D 471/04 (2013.01) [A61K 47/6803 (2017.08); A61K 47/6855 (2017.08); A61P 35/00 (2018.01)] | 17 Claims |
|
1. A compound of formula (XV):
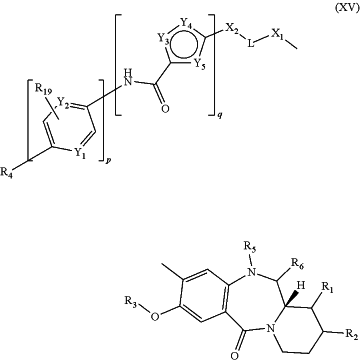 or a pharmaceutically acceptable salt thereof,
wherein:
R1 is O(CH2)nC(O)NHR7, O(CH2)nNHC(O)R7, or R7;
R7 is H, CH3, CH2CH3, CH2CH2CH3, CH(CH3)2, CH2CH2CH2CH3, CH(CH3)CH2CH3, CH2CH(CH3)2, or C(CH3)3;
each n is independently 1, 2, 3, 4, 5, or 6;
R2 is O(CH2) s C(O)NHR9, O(CH2) s NHC(O)R9, or R9;
R9 is H, CH3, CH2CH3, CH2CH2CH3, CH(CH3)2, CH2CH2CH2CH3, CH(CH3)CH2CH3, CH2CH(CH3)2, or C(CH3)3;
each s is independently 1, 2, 3, 4, 5, or 6;
R3 is C1-6 alkyl;
(i) R5 is H; and
R6 is OH or OC1-6 alkyl; or
(ii) R5 and R6, taken together with the N atom and C atom to which they are attached, form N═C;
X1 is —CH2—, —CH2O—, —C(O)—, —C(O)NH—, —C(O)O—, —NH—, —NHC(O)—, —O—, —OC(O)—, or —S—;
L is —C1-12 alkylene-, —(OCH2)1-12—, or —(OCH2CH2)1-6—;
wherein the —C1-12 alkylene optionally contains one or more C—C double bonds or C—C triple bonds; and
wherein the —C1-12 alkylene-, —(OCH2)1-12—, or —(OCH2CH2)1-6— is optionally interrupted by one or more atoms or groups independently selected from the group consisting of NH, O, S, phenylene, and C5-9 heteroarylene;
X2 is absent, —CH2—, —C(O)—, —C(O)NR15—, or —NR15C(O)—;
R15 is H or C1-6 alkyl;
Y3 is —NR17—;
R17 is H or C1-6 alkyl;
Y4 is CH;
Y5 is CH or N;
q is 1;
Y1 is CH or N;
Y2 is CH or N;
R19 is H or (CH2)tNR20R21;
R20 is H or C1-6 alkyl;
R21 is H or C1-6 alkyl;
p is 0;
t is 0, 1, 2, 3, 4, 5, or 6;
R4 is phenyl, furanyl, thiophenyl, oxazolyl, thiazolyl, pyridyl, benzofuranyl, benzothiophenyl, benzimidazolyl, benzoxazolyl, or benzothiazolyl, wherein the phenyl, furanyl, thiophenyl, oxazolyl, thiazolyl, pyridyl, benzofuranyl, benzothiophenyl, benzimidazolyl, benzoxazolyl, or benzothiazolyl is optionally substituted with 1, 2, or 3 substituents independently selected from the group consisting of C1-6 alkyl, (CH2)jC(O)OR11, (CH2)11NR R12, C(O)NH(CH2)kC(NH)NR11R12, C(O)NH(CH2)kNR11R12, C(O)NHR24, OH, OC1-6 alkyl, and O(CH2)kNR11R12;
each R11 is independently H or C1-6 alkyl;
each R12 is independently H or C1-6 alkyl;
each R24 is phenyl, wherein each phenyl is optionally and independently substituted with 1, 2, or 3 independently selected (CH2)jR18 substituents;
each R18 is independently C(O)OR11 or NR11R12;
each j is independently 0, 1, 2, 3, 4, 5, or 6; and
each k is independently 1, 2, 3, 4, 5, or 6.
|
|
14. A pharmaceutical composition comprising a pharmaceutically acceptable excipient, carrier, or diluent and a compound of claim 1, or a pharmaceutically acceptable salt thereof.
|