| CPC C07D 405/12 (2013.01) [C07D 307/89 (2013.01)] | 17 Claims |
|
1. A method for producing an aromatic dianhydride, the method comprising
reacting an aromatic diimide with a substituted or unsubstituted phthalic anhydride in an aqueous medium in the presence of an amine exchange catalyst under conditions effective to provide an aqueous reaction mixture comprising an N-substituted phthalimide, an aromatic tetraacid salt, and at least one of an aromatic triacid salt and an aromatic imide diacid salt, wherein the reacting is at a reaction temperature that is 140 to 250° C. and a reaction pressure of 1.13 to 2.16 MPa;
removing the phthalimide from the aqueous reaction mixture by extracting the aqueous reaction mixture with an organic solvent in a single packed extraction column comprising a structured metal v-shaped packing; and
converting the aromatic tetraacid salt to the corresponding aromatic dianhydride;
wherein
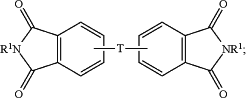
the aromatic diimide is of the formula
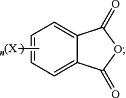
 the substituted or unsubstituted phthalic anhydride is of the formula
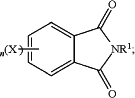
 the N-substituted phthalimide is of the formula
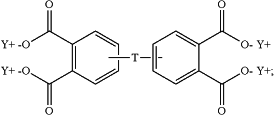
 the aromatic tetraacid salt is of the formula
 the aromatic triacid salt is of the formula
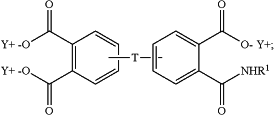 and the aromatic imide diacid salt is of the formula
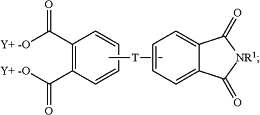 and
the aromatic dianhydride is of the formula
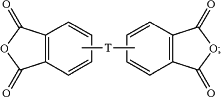 wherein in the forgoing formulas
T is —O—, —S—, —C(O)—, —SO2—, —SO—, —CyH2y— wherein y is an integer from 1 to 5 or a halogenated derivative thereof or —O—Z—O—, wherein Z is an aromatic C6-24 monocyclic or polycyclic moiety optionally substituted with 1 to 6 C1-8 alkyl groups, 1 to 8 halogen atoms, or a combination comprising at least one of the foregoing;
R1 is a monovalent C1-13 organic group;
X is fluoro, chloro, bromo, iodo, or nitro;
n is 0 or 1; and
Y is a cationic group or a proton.
|