| CPC A61N 1/059 (2013.01) [A61N 1/0504 (2013.01); A61N 1/0573 (2013.01); A61N 1/32 (2013.01); A61N 1/37518 (2017.08); A61N 1/40 (2013.01)] | 28 Claims |
|
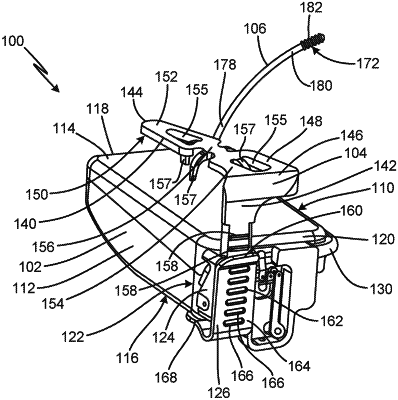
1. A subcutaneously implantable device comprising:
a housing;
a clip attached to the housing that is configured to anchor the device to a muscle and/or a bone, wherein the clip has an anchoring portion that extends along a top side of the housing and a mast portion that extends away from a back end of the anchoring portion and along a back end of the housing, wherein the anchoring portion and the mast portion are configured to move up and down with respect to the housing to increase or decrease an opening between the housing and the anchoring portion of the clip to anchor the device to the muscle and/or the bone;
a first stiff prong with a proximal end attached to the housing and a distal end extending away from the housing that is configured to be positioned adjacent to a first blood vessel;
a first electrode on the distal end of the first stiff prong that is configured to be positioned adjacent to the first blood vessel; and
circuitry in the housing in electrical communication with the first electrode that is configured to deliver electrical stimulation using the first electrode to create an electric field around the first blood vessel.
|