| CPC A61M 5/002 (2013.01) [A61B 6/481 (2013.01); A61B 10/0045 (2013.01); A61M 5/007 (2013.01); A61M 5/365 (2013.01); A61M 39/10 (2013.01); A61M 2005/1403 (2013.01); A61M 2005/3128 (2013.01); A61M 2205/502 (2013.01)] | 12 Claims |
|
1. An injection setup kit comprising:
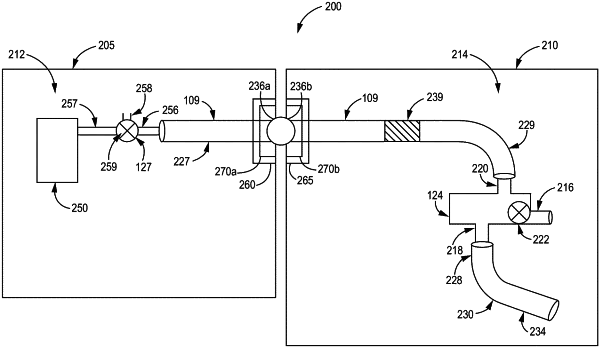
a first packaging container defining a first closed sterile interior volume that comprises:
a patient interface connector including a patient interface inlet, a patient interface outlet, and a valve that is configured to selectively permit fluid to be communicated through the patient interface connector from the patient interface inlet to the patient interface outlet; and
a fluid collection receptacle fluidly connected to the patient interface outlet;
a second packaging container separate from the first packaging container, the second packaging container defining a second closed sterile interior volume that comprises a manifold connector including a manifold inlet and a manifold outlet; and
a fluid line having a first fluid line end and a second fluid line end, wherein the first fluid line end is fluidly connected to the patient interface inlet within the first closed sterile interior volume of the first packaging container, and the second fluid line end is fluidly connected to the manifold outlet within the second closed sterile interior volume of the second packaging container;
wherein:
the first and second packaging containers comprise respective apertures through which the fluid line passes;
the second packaging container is independently openable with respect to the first packaging containers such that when the second packaging container is opened, the first packaging container maintains its first closed sterile interior volume; and
the fluid collection receptacle is configured to hold fluid output from the manifold outlet during an air purge process that is performed when the second packaging container is opened and the first packaging container maintains its first closed sterile interior volume.
|