| CPC A61L 27/18 (2013.01) [A61F 2/08 (2013.01); A61L 27/56 (2013.01); A61L 27/58 (2013.01); A61L 31/06 (2013.01); A61L 31/146 (2013.01); A61L 31/16 (2013.01); B32B 5/18 (2013.01); B32B 27/065 (2013.01); B32B 27/40 (2013.01); C08G 18/10 (2013.01); C08G 18/222 (2013.01); C08G 18/4277 (2013.01); C08G 18/73 (2013.01); C08J 5/18 (2013.01); C08J 9/228 (2013.01); A61F 2002/0894 (2013.01); A61F 2210/0076 (2013.01); A61L 2430/30 (2013.01); A61L 2430/34 (2013.01); B32B 2250/03 (2013.01); B32B 2250/24 (2013.01); B32B 2266/0278 (2013.01); B32B 2266/104 (2016.11); B32B 2307/54 (2013.01); B32B 2307/5825 (2013.01); B32B 2307/7163 (2013.01); B32B 2307/732 (2013.01); B32B 2535/00 (2013.01); C08J 2375/06 (2013.01)] | 30 Claims |
|
1. A tissue repair laminate comprising:
(a) two or more biodegradable polyurethane foam layers; and
(b) one or more structural layers;
wherein the one or more structural layers each have a thickness in the range of from about 50 μm to about 500 μm, and are positioned between said foam layers;
wherein the one or more structural layers consist essentially of biodegradable thermoplastic polyurethane and one or more apertures;
wherein said foam layers comprise a pore structure configured for cellular infiltration, and the foam of said foam layers is a non-reticulated foam;
wherein said tissue repair laminate shrinks due to formation of tissue less than 20% in any one surface area after 20 days under in vivo conditions following implantation of the tissue repair laminate;
and wherein the structural layer polyurethane is derived from components including
one or more chain extenders represented by formula (1) or formula (2)
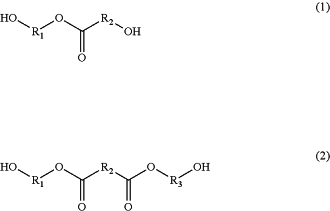 wherein R1, R2 and R3 are independently selected from optionally substituted C1-20 alkylene and optionally substituted C2-20 alkenylene;
one or more aliphatic polyester polyols; and
one or more aliphatic diisocyanates.
|