| CPC A61K 39/12 (2013.01) [A61K 9/0019 (2013.01); A61K 2039/53 (2013.01); A61K 2039/55555 (2013.01)] | 13 Claims |
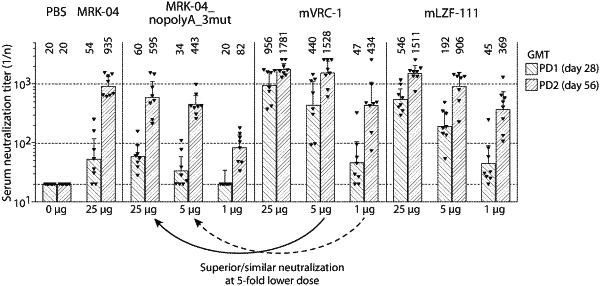
|
1. A respiratory syncytial virus (RSV) messenger ribonucleic acid (mRNA) vaccine composition, comprising:
an mRNA that comprises an open reading frame (ORF) encoding an RSV F protein comprising an amino acid sequence having at least 95% identity to the amino acid sequence of SEQ ID NO: 5, wherein identity defines the percentage of amino acid residues in the RSV F protein that are identical to residues of SEQ ID NO: 5 after introducing any necessary gaps to align the two full-length sequences; and
a lipid nanoparticle comprising 20-60 mol % ionizable cationic lipid, 5-25 mol % neutral lipid, 25-55 mol % sterol, and 0.5-15 mol % PEG-modified lipid.
|