| CPC A61K 31/428 (2013.01) [A61K 9/006 (2013.01); A61K 9/1617 (2013.01); A61K 9/2004 (2013.01); A61K 31/454 (2013.01); A61K 31/496 (2013.01); A61K 31/5377 (2013.01); A61K 38/05 (2013.01); A61K 38/06 (2013.01); A61K 45/06 (2013.01); A61P 25/00 (2018.01); A61P 25/28 (2018.01); C07D 277/82 (2013.01); C07D 417/12 (2013.01); C07K 5/06026 (2013.01); C07K 5/0806 (2013.01); C07K 5/0808 (2013.01); C07K 5/0812 (2013.01); A61K 9/0056 (2013.01); A61K 9/2018 (2013.01); A61K 9/2063 (2013.01); A61K 9/485 (2013.01); A61K 9/4858 (2013.01); A61K 9/4866 (2013.01); A61K 2300/00 (2013.01)] | 9 Claims |
|
1. A pharmaceutical composition comprising:
(i) compound having formula (I):
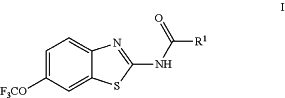 including enantiomers, diastereomers, hydrates, solvates, pharmaceutically acceptable salts, and complexes thereof, wherein:
R1 is CR10aR10bNR11R12,
R10a and R10b are hydrogen,
R11 is hydrogen,
R12 is COCR13aR13bNR15aR15b,
R13a and R13b are hydrogen,
R15a is hydrogen, and
R15b is C1-C6 alkyl; and
(ii) a pharmaceutically acceptable carrier.
|