| CPC A61K 31/231 (2013.01) [A61K 31/14 (2013.01); A61K 31/20 (2013.01); A61K 39/00 (2013.01); A61K 39/0011 (2013.01); A61K 39/12 (2013.01); A61K 39/39 (2013.01); A61K 45/06 (2013.01); A61K 47/646 (2017.08); C07C 219/06 (2013.01); A61K 2039/55555 (2013.01)] | 21 Claims |
|
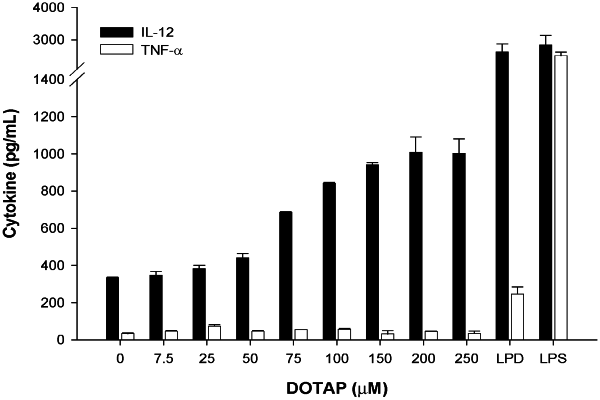
1. A pharmaceutical formulation comprising a liposome together with at least one disease-associated antigen and a non-ionic buffer, wherein the liposome comprises one or more cationic lipids,
wherein the one or more cationic lipid consists of a quaternary cationic lipid, and
wherein the pharmaceutical formulation is free of phosphate buffered saline (PBS); and
wherein the disease-associated antigen is a protein or peptide.
|