| CPC A61B 5/367 (2021.01) [A61B 5/282 (2021.01); A61B 5/339 (2021.01); A61B 5/6823 (2013.01); A61B 5/7475 (2013.01)] | 27 Claims |
|
1. A system comprising:
an electrode apparatus comprising a plurality of external electrodes to be disposed proximate a patient's skin, wherein the plurality of external electrodes comprise a plurality of left external electrodes positioned to the left side of the patient's torso; and
a computing apparatus comprising processing circuitry and coupled to the electrode apparatus and configured to:
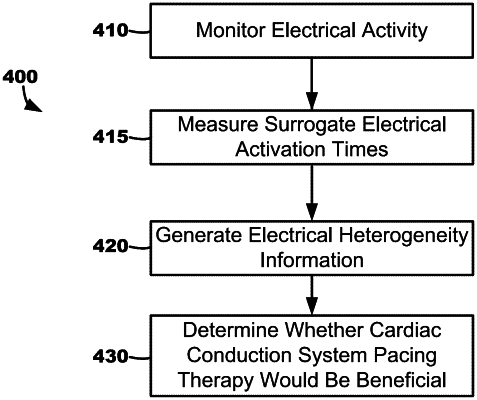
measure surrogate cardiac electrical activation times using the plurality external electrodes of the electrode apparatus during intrinsic activation of the patient's heart, wherein the surrogate cardiac electrical activation times are representative of depolarization of cardiac tissue that propagates through the torso of the patient,
generate electrical heterogeneity information (EHI) based on the measured surrogate cardiac electrical activation times, wherein the EHI comprises one or more left metrics generated based on left-sided activation times of the surrogate cardiac electrical activation times measured using the plurality of left external electrodes, and
determine whether a cardiac conduction system pacing therapy would benefit the patient based on at least the one or more left metrics.
|