| CPC H01L 24/02 (2013.01) [H01L 21/76898 (2013.01); H01L 23/481 (2013.01); H01L 24/06 (2013.01); A61N 1/3754 (2013.01); H01L 24/05 (2013.01); H01L 24/13 (2013.01); H01L 2224/02371 (2013.01); H01L 2224/02372 (2013.01); H01L 2224/02381 (2013.01); H01L 2224/05024 (2013.01); H01L 2224/05569 (2013.01); H01L 2224/05573 (2013.01); H01L 2224/06182 (2013.01); H01L 2224/13026 (2013.01)] | 20 Claims |
|
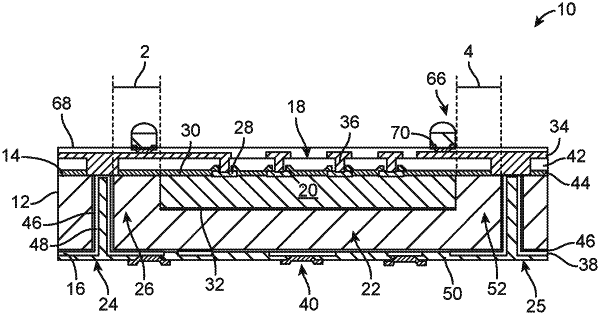
1. An implantable medical device comprising:
a power source;
an electronics module comprising an electronic package that comprises:
a monolithic package substrate comprising a first major surface and a second major surface;
an integrated circuit disposed in an active region of the package substrate; and
a conductive via disposed through an inactive region of the package substrate and extending between the first major surface and the second major surface of the package substrate, wherein the conductive via is separated from the active region by a portion of the inactive region of the substrate; and
a feedthrough header assembly electrically connected to the electronics module.
|