| CPC C40B 40/10 (2013.01) [A61K 38/2013 (2013.01); A61K 38/2026 (2013.01); A61K 38/2086 (2013.01); A61P 35/00 (2018.01); A61P 37/04 (2018.01); C07K 14/5406 (2013.01); C07K 14/5443 (2013.01); C07K 14/55 (2013.01); A61K 48/00 (2013.01); C07K 2319/30 (2013.01)] | 16 Claims |
|
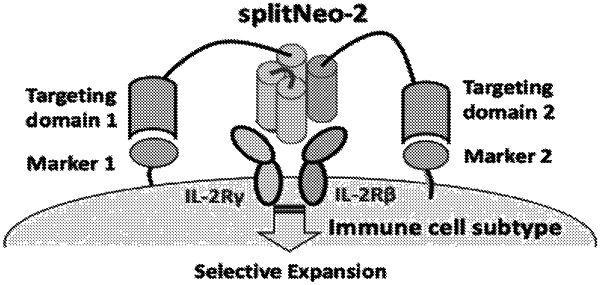
1. A non-naturally occurring conditionally active receptor agonist, comprising a first polypeptide component and a second polypeptide component, wherein the first polypeptide component and the second polypeptide component are not present in a fusion protein, wherein in total the first polypeptide component and the second polypeptide component comprise domains X1, X2, X3, and X4, wherein:
(a) X1 is a peptide comprising an amino acid sequence at least 75% identical to the amino acid sequence (PKKKIQ) LHAEHALYDAL(MILNI) (SEQ ID NO: 4);
(b) X2 is any helical peptide domain;
(c) X3 is a peptide comprising an amino acid sequence at least 75% identical to the amino acid sequence (LE) DYAFNFELILEE(IARLFESG) (SEQ ID NO:5); and
(d) X4 is a peptide comprising an amino acid sequence at least 75% identical to the amino acid sequence (EDEQEEMANAI)ITILQSWIF(S) (SEQ ID NO:6),
wherein:
(i) amino acid residues in parentheses may be present or absent;
(ii) the first polypeptide component comprises at least one of X1, X2, X3, and X4 but does not comprise each of X1, X2, X3, and X4; and
iii) the second polypeptide component comprises each of X1, X2, X3, and X4 that is not present in the first polypeptide component;
wherein the first polypeptide component and the second polypeptide component are not active receptor agonists individually, and wherein the first polypeptide component and the second polypeptide interact to form an active agonist of IL-2 receptor β
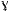 c heterodimer (IL-2Rβ c heterodimer (IL-2Rβ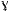 c), or IL-4 receptor α c), or IL-4 receptor α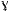 c heterodimer (IL-4Rα c heterodimer (IL-4Rα c). c). |