| CPC C11D 17/045 (2013.01) [B31B 70/74 (2017.08); B65D 29/00 (2013.01); C11D 17/042 (2013.01); B31B 2160/20 (2017.08)] | 17 Claims |
|
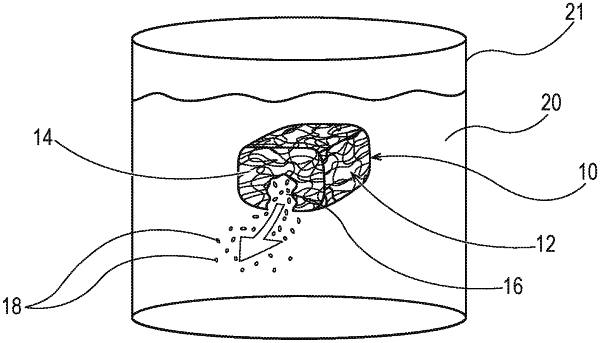
1. A water-soluble unit dose product, wherein the unit dose product comprises a pouch comprising a water-soluble fibrous wall material comprising a plurality of fibrous elements comprising one or more filament-forming materials comprising polyvinyl alcohol and one or more active agents present within the fibrous elements, wherein the water-soluble fibrous wall material defines an internal volume of the pouch, wherein one or more active agents in particle form are present within the internal volume of the pouch, wherein at least one of the one or more active agents in particle form is released from the internal volume of the pouch when the unit dose product ruptures when exposed to conditions of intended use, and wherein the one or more active agents within the internal volume of the pouch comprise one or more of the following: personal cleansing agents, personal conditioning agents, hair care agents, hair colorant agents, hair conditioning agents, skin care agents, sunscreen agents, skin conditioning agents and mixtures thereof;
wherein the unit dose product exhibits an Average Rupture Time of less than 240 seconds as measured according to the Rupture Test Method;
wherein the unit dose product exhibits a % Weight Loss of less than 10% as measured according to the Shake Test Method;
wherein the fibrous wall material exhibits a Geometric Mean (GM) Tensile Strength of greater than 0.1 kN/m, and a Geometric Mean (GM) Elongation at Break of less than 1000%.
|