| CPC C07J 71/0031 (2013.01) [A61K 31/58 (2013.01); A61K 31/7048 (2013.01); A61K 45/06 (2013.01); C07J 71/0005 (2013.01)] | 17 Claims |
|
1. A method for reducing beta-amyloid peptide production in a host, comprising administering to a host a compound of Formula I:
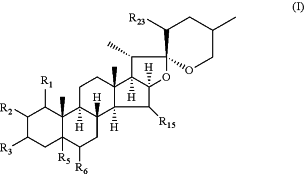 or a single enantiomer, a mixture of an enantiomeric pair, an individual diastereomer, or a mixture of diastereomers; or a pharmaceutically acceptable salt, solvate, or prodrug thereof, wherein
each of R1, R2, R15, and R23 is independently H, OH, oxo, O-acyl, halo, O-alkyl, OSO3H, OSO3Na, N3, NH2, NHR, NRR′, NHSO2R, SR, SOR, SO2R, OPO3H2, OPO3HNa, OPO3Na2, O-monosaccharide, O-disaccharide or O-oligosaccharide;
R3 is O-alkyl;
wherein the alkyl in R3 is substituted by —C(O)ORa wherein Ra is hydrogen, or C3-20-alkyl; or
wherein the alkyl in R3 is substituted by —C(O)NRbRc, and wherein Rb and Rc are each independently hydrogen or alkyl, wherein the alkyl is optionally substituted with heterocyclyl which is optionally substituted with one or more —ORa substituents where Ra is hydrogen or alkyl; and
each of R5 and R6 is independently H, OH, O-acyl, halo, OSO3H, O-alkyl, OSO3H, OSO3Na, N3, NH2, NHR, NRR′, NHS2R, SR, SOR, SO2R, OPO3H2, OPO3HNa, OPO3Na2, epoxy, O-monosaccharide, O-disaccharide or O-oligosaccharide, or R5 and R6 together form a double bond,
where each of R and R′ independently is alkyl, aryl, arylalkyl, alkaryl, heteroaryl, alkenyl, alkynyl, cycloalkyl, or heterocyclyl;
wherein each monosaccharide in R1, R2, R15, R23, R5, and R6 is independently glucose, fructose, galactose, xylose, ribose, or mannose;
wherein each disaccharide in R1, R2, R15, R23, R5, and R6 is independently sucrose, lactose, maltose, trehalose, or cellobiose; and
wherein each oligosaccharide in R1, R2, R15, R23, R5, and R6 is independently starch, glycogen, cellulose, chitin, and combinations thereof.
|