| CPC C07D 498/04 (2013.01) [A61P 31/04 (2018.01); A61P 31/12 (2018.01); A61P 31/14 (2018.01); A61P 31/16 (2018.01); A61P 31/18 (2018.01); C07D 231/18 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07D 513/04 (2013.01)] | 19 Claims |
|
1. A compound of Formula (I-5),
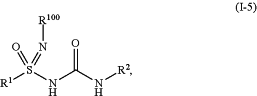 or a pharmaceutically acceptable salt thereof, wherein:
R100 is selected from the group consisting of H, Cl, D, —CN, —NO2, —OR3a, —C(O)R3b, —S(O)2R3b, —S(O)R3b, C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6-membered heteroaryl; wherein the C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of D, —CN, halogen, C1-C6alkyl, —OR3a, —C(O)R3b, —P(O)R3bR4b, —S(O)2R3b, —S(O)R3b, —NR3aR4a, —NR3aC(O)R4a, —NR3aC(O)OR4a, —NR3aC(O)NR4a, —NR3aS(O)2R4a, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl;
R1 is selected from the group consisting of
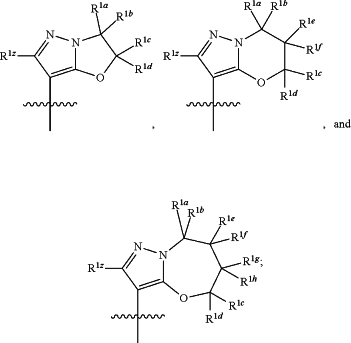 wherein R1z is H, D, halogen, —CN, —NO2, —SR7a, —OR7a, —C(O)R7b, —P(O)R7bR8b, —S(O)2R7b, —S(O)R7b, —NR7aR8a, —NR7aC(O)R8a, —NR7aC(O)OR8a, —NR7aC(O)NR8a, —NR7aS(O)2R8a, C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, or 5-6-membered heteroaryl; wherein the C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of D, —CN, halogen, C1-C6alkyl, —OR7a, —C(O)R7b, —P(O)R7bR8b, —S(O)2R7b, —S(O)R7b, —NR7aR8a, —NR7aC(O)R8a, —NR7aC(O)OR8a, —NR7aC(O)NR8a, —NR7aS(O)2R8a, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl;
each R1a, R1b, R1c, R1d, R1e, R1f, R1g, and R1h is independently selected from the group consisting of H, D, halogen, —CN, —NO2, —SR11a, —OR11a, —C(O)R11b, —P(O)R11bR12b, —S(O)2R11b, —S(O)R11b, —NR11aR12a, —NR11aC(O)R12a, —NR11aC(O)OR12a, —NR11aC(O)NR12a, —NR11aS(O)2R12a, C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6-membered heteroaryl; wherein the C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of D, —CN, halogen, C1-C6alkyl, —OR11a, —C(O)R11b, —P(O)R11bR12b, —S(O)2R11b, —S(O)R11b, —NR11aR12a, —NR11aC(O)R12a, —NR11aC(O)OR12a, —NR11aC(O)NR12a, —NR11aS(O)2R12a, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl; or
two of the following groups, R1a, R1b, R1c, R1d, R1e, R1f, R1g, and R1h, when present, together with the atoms to which they are attached can form a C3-C10cycloalkyl or a 3-7-membered heterocyclyl; wherein the C3-C10cycloalkyl and 3-7-membered heterocyclyl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of D, —CN, halogen, C1-C6alkyl, —OR13a, —C(O)R13b, —P(O)R13bR14b, —S(O)2R13b, —S(O)R13b, —NR13aR14a, —NR13aC(O)R14a, —NR13aC(O)OR14a, —NR13aC(O)NR14a, and —NR13aS(O)2R14a, or
two geminal groups R1a and R1b, R1c and R1d, R1e and R1f; or R1g and R1h, when present, can form an oxo group;
R2 is
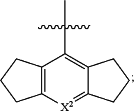 X2 is N or CR2a;
X3 is N or CR2e;
X4 is N or CR2d;
X5 is N or CR2e;
X6 and X7 are independently N or CR2n, wherein at least one of X6 and X7 is N;
R2a is H, D, halogen, —CN, —OR15a, C1-C6alkyl, C3-C10cycloalkyl, —C(O) NR15aR16a, —C(O)OR15a, —NR15aR16a, —NR15aC(O)R16a, —NR15aC(O)OR16a, —NR15aC(O)NR16a, or —NR15aS(O)2R16a, wherein the C1-C6alkyl and C3-C10cycloalkyl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of D, halogen, —CN, —OR15a, —C(O)R15b, —NR15aR16a, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl;
each R2b, R2c, R2d, R2e, and R2f is independently H, D, halogen, —CN, —NO2, —SR17a, —OR17a, —C(O)R17b, —P(O)R17bR18b, —S(O)2R17b, —S(O)R17b, —NR17aR18a, —NR17aC(O)R18a, —NR17aC(O)OR18a, —NR17aC(O)NR18a, —NR17aS(O)2R18a, C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, or 5-6 membered heteroaryl; wherein the C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of D, —CN, halogen, —OR17a, —C(O)R17b, —P(O)R17bR18b, —S(O)2R17b, —S(O)R17b, —NR17aR18a, —NR17aC(O)R18a, —NR17aC(O)OR18a, —NR17aC(O)NR18a, —NR17aS(O)2R18a, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl; or
two adjacent R2b, R2c, R2d, R2e, and R2f together with the atoms to which they are attached can form C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, or 5-6-membered heteroaryl; wherein the C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6-membered heteroaryl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of halogen, D, —CN, C1-C6alkyl, —OR19a, and NR19aR20a;
each R2j, R2k, R2m, and R2n is independently H, D, halogen, —CN, —NO2, —SR17a, —OR17a, —C(O)R17b, —P(O)R17bR18b, —S(O)2R17b, —S(O)R17b, —NR17aR18a, —NR17aC(O)R18a, —NR17aC(O)OR18a, —NR17aC(O)NR18a, —NR17aS(O)2R18a, C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, or 5-6 membered heteroaryl; wherein the C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of D, —CN, halogen, —OR17a, —C(O)R17b, —P(O)R17bR18b, —S(O)2R17b, —S(O)R17b, —NR17aR18a, —NR17aC(O)R18a, —NR17aC(O)OR18a, —NR17aC(O)NR18a, —NR17aS(O)2R18a, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl; or
two adjacent R2j, R2k, R2m, and R2n together with the atoms to which they are attached can form C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, or 5-6-membered heteroaryl; wherein the C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6-membered heteroaryl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of halogen, D, —CN, C1-C6alkyl, —OR19a, and NR19aR20a;
each R2g and R2h is independently H, C1-C6alkyl, C3-C10cycloalkyl, 3-7-membered heterocyclyl, C6aryl, or 5-membered heteroaryl, wherein the 3-7-membered heterocyclyl and 5-membered heteroaryl are attached to the nitrogen at a carbon on the 3-7-membered heterocyclyl or 5-membered heteroaryl, and wherein the C1-C6alkyl, C3-C10cycloalkyl, 3-7-membered heterocyclyl, C6aryl, or 5-membered heteroaryl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of D, —CN, halogen, C1-C6alkyl, —OR21a, —C(O)R21b, —P(O)R21bR22b, —S(O)2R21b, —S(O)R21b, —NR21aR22a, —NR21aC(O)R22a, —NR21aC(O)OR22a, —NR21aC(O)NR22a, —NR21aS(O)2R22a, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-membered heteroaryl;
R3a, R4a, R7a, R8a, R11a, R12a, R13a, R14a, R15a, R16a, R17a, R18a, R19a, R20a R21a, R22a, R23a, and R24a are independently, at each occurrence, H, D, C1-C8alkyl, C2-C8alkenyl, C2-C8alkynyl, C3-C10cycloalkyl, C4-C8cycloalkenyl, C6aryl, 3-7-membered heterocyclyl, or 5-6-membered heteroaryl; wherein the C1-C8alkyl, C2-C8alkenyl, C2-C8alkynyl, C3-C10cycloalkyl, C4-C8cycloalkenyl, C6aryl, 3-7-membered heterocyclyl, and 5-6-membered heteroaryl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of D, —CN, halogen, C1-C6alkyl, —OH, —O—C1-C6alkyl, —NH2, —NH(C1-C6alkyl), —N(C1-C6alkyl)2, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl;
R3b, R4b, R7b, R8b, R11b, R12b, R13b, R14b, R15b, R17b, R18b, R21b, R22b, R23b, and R24b are independently, at each occurrence, H, D, —OH, —O(C1-C6alkyl), —NH2, —NH(C1-C6alkyl), —N(C1-C6alkyl)2, —NHS(O)2CH3, C1-C6alkyl, C2-C8alkenyl, C2-C8alkynyl, C3-C10cycloalkyl, C4-C8cycloalkenyl, C6aryl, 3-7-membered heterocyclyl, or 5-6-membered heteroaryl; wherein the C1-C8alkyl, C2-C8alkenyl, C2-C8alkynyl, C3-C10cycloalkyl, C4-C8cycloalkenyl, C6aryl, 3-7-membered heterocyclyl, and 5-6-membered heteroaryl are independently unsubstituted or substituted with one or more substituents selected from the group consisting of D, —CN, halogen, C1-C6alkyl, —OH, —O—C1-C6alkyl, —NH2, —NH(C1-C6alkyl), —N(C1-C6alkyl)2, C3-C10cycloalkyl, C6aryl, 3-7-membered heterocyclyl, and 5-6 membered heteroaryl.
|