| CPC C07D 487/04 (2013.01) [A61K 9/2009 (2013.01); A61K 9/2013 (2013.01); A61K 9/2018 (2013.01); A61K 9/2027 (2013.01); A61K 9/2054 (2013.01); A61K 9/2059 (2013.01); A61K 9/485 (2013.01); A61K 9/4858 (2013.01); A61K 9/4866 (2013.01); A61K 31/495 (2013.01); A61K 47/02 (2013.01); A61K 47/24 (2013.01); A61K 47/26 (2013.01); A61K 47/34 (2013.01); A61K 47/36 (2013.01)] | 13 Claims |
|
1. A compound of Formula (I):
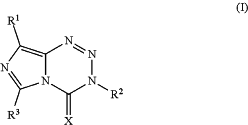 or a pharmaceutically acceptable salt thereof,
wherein:
R1 is 5- or 6-membered heterocycloalkyl, wherein the 5- or 6-membered heterocycloalkyl is optionally substituted with one or more substituents independently selected from the group consisting of F, Cl, Br, I, CN, NO2, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, (CH2)1-2NHC(O)R′, C(NH)N(R′)2, C(NOR′)R′, C(O)R′, C(O)CH2C(O)R′, C(O)C(O)R′, C(O)N(R′)2, C(O)N(OR)R′, C(O)OR', C(S)R′, C(S)N(R′)2, N(R′)2, NR′C(O)R′, NR′C(O)N(R′)2, NR′C(O)OR″, NR′C(S)R′, NR′C(S)(R′)2, NR′NR′C(O)R′, NR′NR′CON(R′)2, NR′NR′C(O)OR', NR′S(O)2R′, NR′S(O)2N(R′)2, N[C(O)R′]C(O)R′, N(OR′)R′, OR′, OCF3, —OCH2O—, —OCH2CH2O—, OC(O)R′, OC(O)N(R′)2, ═O, SR′, S(O)R′, S(O)2R′, S(O)2N(R′)2, S(O)2OR′, ═S, and C3-C6 cycloalkyl;
R2 is C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, or C3-C6 cycloalkyl;
wherein the C1-C6 alkyl, C2-C6 alkenyl, or C2-C6 alkynyl is optionally substituted with one or more substituents independently selected from the group consisting of F, Cl, Br, I, CN, NO2, (CH2)1-2NHC(O)R′, C(NH)N(R′)2, C(NOR′)R′, C(O)R′, C(O) CH2C(O)R′, C(O)C(O)R′, C(O)N(R′)2, C(O)N(OR)R′, C(O)OR′, C(S)R′, C(S)N(R′)2, N(R′)2, NR′C(O)R′, NR′C(O)N(R′)2, NR′C(O)OR', NR′C(S)R′, NR′C(S)N(R′)2, NR′NR′C(O)R′, NR′NR′CON(R′)2, NR′NR′C(O)OR′, NR′S(O)2R′, NR′S(O)2N(R′)2, N[C(O)R′]C(O)R′, N(OR′)R′, OR′, OCF3, —OCH2O—, —OCH2CH2O—, OC(O)R′, OC(O)N(R′)2, SR′, S(O)R′, S(O)2R′, S(O)2N(R′)2, S(O)2OR′, and C3-C6 cycloalkyl; and
wherein the C3-C6 cycloalkyl is optionally substituted with one or more substituents independently selected from the group consisting of F, Cl, Br, I, CN, NO2, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, (CH2)1-2NHC(O)R′, C(NH)N(R′)2, C(NOR′)R′, C(O)R′, C(O)CH2C(O)R′, C(O)C(O)R′, C(O)N(R′)2, C(O)N(OR)R′, C(O)OR′, C(S)R′, C(S)N(R′)2, N(R′)2, NR′C(O)R′, NR′C(O)N(R′)2, NR′C(O)OR', NR′C(S)R′, NR′C(S)N(R′)2, NR′NR′C(O)R′, NR′NR′CON(R′)2, NR′NR′C(O)OR′, NR′S(O)2R′, NR′S(O)2N(R′)2, N[C(O)R′]C(O)R′, N(OR′)R′, OR′, OCF3, —OCH2O—, —OCH2CH2O—, OC(O)R′, OC(O)N(R′)2, ═O, O(phenyl), SR′, S(O)R′, S(O)2R′, S(O)2N(R′)2, S(O)2OR′, ═S, and C3-C6 cycloalkyl;
R3 is H, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, or C3-C6 cycloalkyl;
wherein the C1-C6 alkyl, C2-C6 alkenyl, or C2-C6 alkynyl is optionally substituted with one or more substituents independently selected from the group consisting of F, Cl, Br, I, CN, NO2, (CH2)1-2NHC(O)R′, C(NH)N(R′)2, C(NOR′)R′, C(O)R′, C(O)CH2C(O)R′, C(O)C(O)R′, C(O)N(R′)2, C(O)N(OR)R′, C(O)OR′, C(S)R′, C(S)N(R′)2, N(R′)2, NR′C(O)R′, NR′C(O)N(R′)2, NR′C(O)OR′, NR′C(S)R′, NR′C(S)N(R′)2, NR′NR′C(O)R′, NR′NR′CON(R′)2, NR′NR′C(O)OR′, NR′S(O)2R′, NR′S(O)2N(R′)2, N[C(O)R′]C(O)R′, N(OR′)R′, OR′, OCF3, —OCH2O—, —OCH2CH2O—, OC(O)R′, OC(O)N(R′)2, SR′, S(O)R′, S(O)2R′, S(O)2N(R′)2, S(O)2OR′, and C3-C6 cycloalkyl; and
wherein the C3-C6 cycloalkyl is optionally substituted with one or more substituents independently selected from the group consisting of F, Cl, Br, I, CN, NO2, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, (CH2)1-2NHC(O)R′, C(NH)N(R′)2, C(NOR′)R′, C(O)R′, C(O)CH2C(O)R′, C(O)C(O)R′, C(O)N(R′)2, C(O)N(OR)R′, C(O)OR′, C(S)R′, C(S)N(R′)2, N(R′)2, NR′C(O)R′, NR′C(O)N(R′)2, NR′C(O)OR′, NR′C(S)R′, NR′C(S)N(R′)2, NR′NR′C(O)R′, NR′NR′CON(R′)2, NR′NR′C(O)OR′, NR′S(O)R′, NR′S(O)2N(R′)2, N[C(O)R′]C(O)R′, N(OR′)R′, OR′, OCF3, —OCH2O—, —OCH2CH2O—, OC(O)R′, OC(O)N(R′)2, ═O,SR′, S(O)R′, S(O)2R′, S(O)2N(R′)2, S(O)2OR′, ═S, and C3-C6 cycloalkyl;
each R′ is independently H, C1-C6 alkyl, C2-C6 alkenyl, or C2-C6 alkynyl; and
X is O or S.
|