| CPC C07D 471/04 (2013.01) | 13 Claims |
|
1. A method for synthesizing 1,7-naphthyridine derivatives, comprising the following steps:
(1) dissolving Compound I, a protecting group reagent and an acid-binding agent in a solvent to make compound II at a reaction temperature of 20-150° C.;
wherein the molar ratio of said Compound I, said protecting group reagent and said acid-binding agent is 1.0:1.0˜10.0:0˜15.0,
wherein the compound I is a starting material and is 2-chloro-3-amino-pyridine;
said protecting group reagent is any one or more of di-tert-butyl dicarbonate, diisobnate, di-n-butyl dicarbonate, dibenzyl dicarbonate, diethyl dicarbonate, dimethyl dicarboutyl dicarbonate, di-n-propyl dicarbonate, diisopropyl dicarbonate, tert-butyl chloroformate, isobutyl chloroformate, n-butyl chloroformate, benzyl chloroformate, methyl chloroformate, ethyl chloroformate, n-propyl chloroformate and isopropyl chloroformate,
the corresponding protective group R1 in said compound II is any one or more of tert-butoxycarbonyl, isobutoxycarbonyl, n-butoxycarbonyl, benzyloxycarbonyl, methoxycarbonyl, ethoxycarbonyl, n-propoxycarbonyl and isopropoxycarbonyl,
said acid-binding agent is any one or more of sodium hydroxide, potassium hydroxide, potassium carbonate, sodium carbonate, triethylamine and N-methylmorpholine;
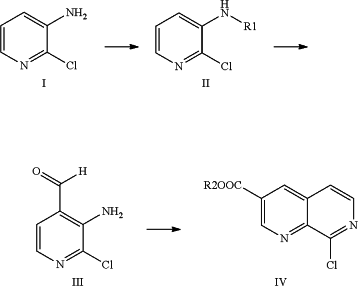 (2) said Compound II is reacted with an aldehydation reagent under alkaline conditions to make Compound III,
wherein said aldehydation reagent is added dropwise, the temperature is ˜100˜10° C., the molar ratio of said Compound II, said alkali, said tetramethylethylene diamine and said aldehydation reagent is 1.0:1.0˜5.0:1.0˜5.0:1.0˜5.0,
said alkali is any one or more of n-butyl lithium, tert-butyl lithium, lithium diisopropylamide, and lithium hexamethyldisilazide,
said solvent is any one or more of tetrahydrofuran, methyltetrahydrofuran, dioxane, and methyl tert-butyl ether,
said aldehydation reagent is any one or more of dimethylformamide, diethylformamide, and N-formylmorpholine;
(3) said Compound III is cyclized with acrylate compounds make Compound IV with a Lewis acid,
wherein said Compound III, said Lewis acid and said acrylate compounds are dissolved in a solvent, and the temperature is 10˜120° C.,
said solvent is any one or more of ethyl acetate, isopropyl acetate, acetonitrile, tetrahydrofuran, methyltetrahydrofuran, dioxane and methyl tert-butyl ether,
said Lewis acid is any one or more of sodium tetrafluoroborate, lithium tetrafluoroborate, calcium tetrafluoroborate and potassium tetrafluoroborate,
said acrylate compounds are N,N-dimethylamino ethyl acrylate, N,N-dimethylamino acrylate, N,N-dimethylamino propyl acrylate and N,N-dimethylamino butyl acrylate,
the molar ratio of said Compound III, said Lewis acid and said acrylate compounds is 1.0:0.5˜10:1.0˜5.0,
R2 in said Compound IV is any one of a hydrogen atom, a methyl group, an ethyl group, a propyl group, an isopropyl group, a butyl group, an isobutyl group and a tert-butyl group.
|