| CPC C07D 471/04 (2013.01) [C07B 2200/13 (2013.01)] | 19 Claims |
|
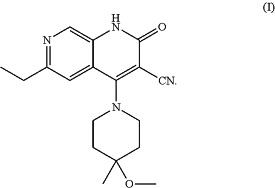
1. A crystal form I of a compound as shown in formula (I), 6-ethyl-4-(4-methoxy-4-methylpiperidin-1-yl)-2-oxo-1,2-dihydro-1,7-diazanaphthalene-3-carbonitrile, wherein the crystal form has an X-ray powder diffraction pattern comprising characteristic peaks at 7.3±0.2°, 13.6±0.2°, 14.5±0.2°, 18.0±0.2°, 19.1±0.2°, 22.0±0.2° and 23.4±0.2° 2θ, by using Cu-Kα radiation,
 |