| CPC C07D 413/14 (2013.01) [C07D 401/14 (2013.01); C07D 403/10 (2013.01); C07D 403/12 (2013.01); C07D 405/14 (2013.01); C07D 471/04 (2013.01)] | 5 Claims |
|
1. A method of producing a modified immune cell, comprising culturing a cell population containing an immune cell in the presence of an effective amount of a Cbl-b inhibitor to modulate activity of the immune cell, thereby producing the modified immune cell, wherein the Cbl-b inhibitor is a compound of Formula (I)
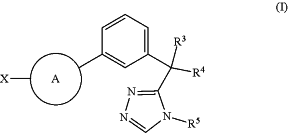 or a tautomer thereof, or a pharmaceutically acceptable salt thereof, wherein
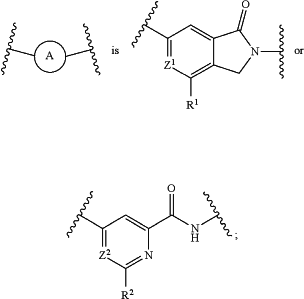 Z1 is CH or nitrogen;
Z2 is CH or nitrogen;
R1 is —CF3 or cyclopropyl;
R2 is —CF3 or cyclopropyl;
R3 is hydrogen, C1-C2 alkyl, or C1-C2 haloalkyl;
R4 is hydrogen, C1-C6 alkyl, C1-C6 haloalkyl, 4- to 8-membered heterocyclyl, or C3-C6 cycloalkyl,
wherein the heterocyclyl or cycloalkyl groups are optionally substituted by one to five R6 groups;
or R3 and R4 are taken together with the carbon atom to which they are attached to form a C3-C5 cycloalkyl or 4- to 6-membered heterocyclyl, each of which is optionally substituted by one to five R6 groups;
R5 is hydrogen, C1-C6 alkyl, C1-C6 haloalkyl, or C3-C6 cycloalkyl;
each R6 is independently C1-C6 alkyl, halo, hydroxy, —O(C1-C6 alkyl), —CN,
C1-C6 alkyl-CN, C1-C6 alkyl-OH, or C1-C6 haloalkyl;
or two R6 groups attached to the same carbon atom are taken together with the carbon atom to which they are attached to form a spiro C3-C6 cycloalkyl or spiro 4- to 6-membered heterocyclyl;
X is hydrogen, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 alkyl-OH, C1-C6 alkyl-CN,
C3-C6 cycloalkyl optionally substituted by one to five R8 groups, or
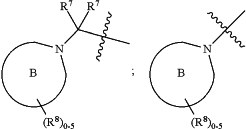 is 4- to 7-membered heterocyclyl or 5- to 8-membered heteroaryl, wherein each heterocyclyl or heteroaryl optionally contains one to two additional heteroatoms selected from the group consisting of nitrogen, oxygen, and sulfur, and wherein each heterocyclyl or heteroaryl is optionally substituted by one to five R8 groups;
each R7 is independently hydrogen, C1-C6 alkyl, C1-C6 alkyl-OH, or C1-C6 haloalkyl;
or two R7 groups are taken together with the carbon atom to which they are attached to form a C3-C5 cycloalkyl or 3- to 5-membered heterocyclyl; and
each R8 is independently halo, C1-C6 alkyl, C1-C6 alkyl-CN, C1-C6 alkyl-OH,
C1-C6 haloalkyl, —CN, oxo, or —O(C1-C6 alkyl);
or two R8 groups are taken together with the carbon atom or atoms to which they are attached to form a spiro or fused C3-C5 cycloalkyl or 3- to 5-membered heterocyclyl.
|