| CPC C07C 29/00 (2013.01) [C07C 37/14 (2013.01); C07C 37/48 (2013.01); C07C 41/01 (2013.01); C07C 67/00 (2013.01); C07C 391/02 (2013.01); C07C 2601/10 (2017.05)] | 5 Claims |
|
1. A process for the preparation of cannabidiol compound of formula (A)
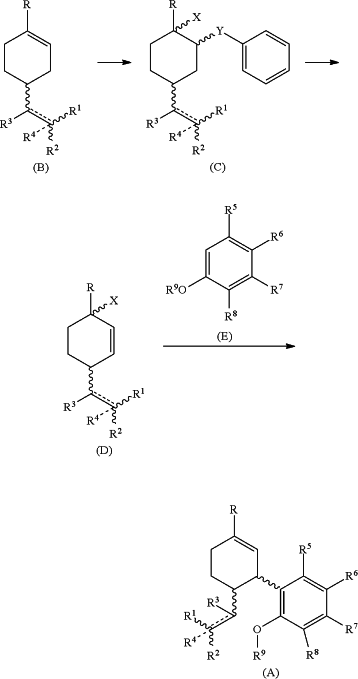 comprising the steps of:
(a) contacting a compound of formula (B) with a reagent selected from PhSeBr, PhSeCl, PhSCI, PhSBr, PhSSPh/AgOTf, PhSSPh/N-Chloromethyl-N′-fluorotriethylenediammonium bis(tetrafluoroborate), PhSeSePh/AgOTf, or PhSeSePh/N-Chloromethyl-N′-fluorotriethylenediammonium bis(tetrafluoroborate),
in the presence of a solvent or a mixture of solvents selected from H2O, tetrahydrofuran, dioxane, acetonitrile, chlorobenzene, dichloroethane, acetone, hexane, dichloromethane, chloroform, ethyl acetate, and toluene; and with stirring for a time period in the range of 0.1 h-48 h and at a temperature in the range of −80° C. to 60° C., whereby a compound of formula (C) is produced;
(b) contacting the compound of formula (C) with an oxidant selected from mCPBA, Oxone, DDQ, CAN, N-hydroxy succinamide, t-Butylhydroperoxide, N-Chloromethyl-N′-fluorotriethylenediammonium bis(tetrafluoroborate), Hydrogen peroxide, BIAB, NFSI, TMSOTf, PyF-BF4, PyF-OTf, TMPyF-OTf, and PIFA,
in the presence of a solvent or a mixture of solvents selected from H2O, tetrahydrofuran, dioxane, acetonitrile, chlorobenzene, dichloroethane, acetone, hexane, dichloromethane, chloroform, ethyl acetate, and toluene; and with stirring the for a time period in the range of 0.1 to 48 hours at a temperature in the range of −40° C. to 60° C., whereby a compound of formula (D) is produced;
(c) contacting the compound of formula (D) with a compound of formula (E) in the presence of
(i) a metal triflate selected from AgOTf, Ni(OTf)2, Hg(OTf)2, LiOTf, Bi(OTf)3, Ln(OTf)3, and Ac(OTf)x, optionally in the presence of a ligand selected from bipyridyl, substituted bipyridyl phenanthrolene, substituted phenanthrolene, pyridine, substituted pyridine, BINAP, QINAP, PINAP, Ph3P; or
(ii) a heterogeneous acid selected from mixed metal oxides, SiO2—SO3H/COFe2O4, SiO2—Pr—SO3H, zeolites, zeotype materials, OMR-[C4HMTA][SO3H], MPD-SO3H-IL, MeAPSO, MeAPO, SAPO, ALPO4, Natrolite, ZSM-5, H-ZSM-5, periodic mesoporous organosilicas (PMOs), mesoporous silicas (PMSs), H3PW12O40, H4SiW12O40, Cs2HPW12O40, HPW/ZrO2, HPW/Nb2O5, Montmorillonite, pyrophyllite, Talc, Vermiculite, Sauconite, Saponite, Nontronite, Kaolinite, Chlorite, Illite, SAPO-34, Zirconium phosphates or sulphates, cation/anion exchange resins, amberlyst, and amberlite;
in the presence of a solvent or mixture of solvents selected from tetrahydrofuran, dioxane, acetonitrile, chlorobenzene, dichloroethane, acetone, hexane, dichloromethane, chloroform, ethyl acetate, and toluene; and with stirring for time period in the range of 0.1 to 48 hours at a temperature in the range of −40° C. to 60° C.;
wherein
R is independently selected from H, OH, alkyl, alkenyl, alkynyl, and cycloalkyl;
R1, R2, R3 and R4 are independently selected from H, OH, alkyl, alkenyl, alkynyl, acyl, acyloxy, and cycloalkyl;
X is independently selected from OH, H, heteroaryl, Cl, Br, I, OTf, OTs, and phosphinyl;
Y is independently selected from S and Se;
wherein, each
 represents a single or double bond; provided that both represents a single or double bond; provided that both  groups are not double bonds, and wherein denoted, dash marks indicate the points of attachment; groups are not double bonds, and wherein denoted, dash marks indicate the points of attachment;wherein,
 represents a single bond, above the plane or below the plane or both above the plane or both below the plane or one is above the plane and one is below the plane. represents a single bond, above the plane or below the plane or both above the plane or both below the plane or one is above the plane and one is below the plane. |