| CPC C07C 227/02 (2013.01) [C07C 255/25 (2013.01)] | 6 Claims |
|
1. A method of making ethylenediaminetetraacetic acid (EDTA) comprising the steps:
(a) providing a reaction mixture (a) comprising ethylenediamine (EDA) and glycolonitrile (GN), wherein reaction mixture (a) comprises 0% to 0.1% by weight, based on the weight of reaction mixture (a), of any base having pKa of the conjugate acid (PKaH) of 13 or higher;
(b) causing or allowing reaction mixture (a) to react to form a dinitrile compound having structure DN:
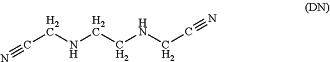 (c) bringing the DN into contact with aqueous solution of a base having pKaH of 11 or higher, and causing or allowing the resulting mixture to react to form a diacid compound having structure DA:
 wherein each —R has the following structure:
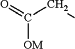 wherein each M is independently hydrogen or an alkali metal;
(d) causing or allowing the DA to react, either sequentially or simultaneously, with additional GN to form products (Pd);
(e) causing or allowing products (Pd) to react with a base having pKaH of 11 or higher to form EDTA.
|