| CPC A61L 2/20 (2013.01) [A61L 2/26 (2013.01); A61L 2202/122 (2013.01); A61L 2202/21 (2013.01); A61L 2202/24 (2013.01)] | 24 Claims |
|
1. A method of externally sterilizing a drug delivery device utilizing Nitrogen Dioxide (NO2), comprising:
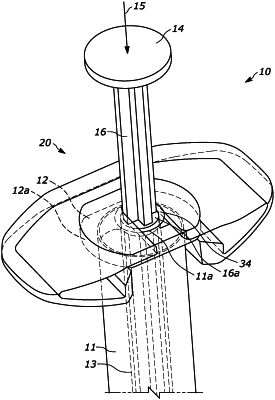
placing the drug delivery device in a sterilization chamber, wherein the drug delivery device is a syringe comprising a barrel, a flange portion, and a backstop coupled with the flange portion;
introducing into the sterilization chamber a dose of NO2 having a concentration of between about 2 and 20 milligrams per Liter by applying a vacuum level of between about 10 and 600 Torr and holding the vacuum level for a dwell time of between about 2 and 20 minutes, wherein the backstop is configured to permit or promote the NO2 to flow through a space between the backstop and the syringe when the backstop is coupled with the syringe;
repeating the step of introducing into the sterilization chamber the dose of NO2 for a number of pulses between about 1 and 24;
purging the sterilization chamber of at least substantially all of the NO2; and
aerating the sterilization chamber.
|