| CPC A61K 8/494 (2013.01) [A61K 8/355 (2013.01); A61K 8/49 (2013.01); A61Q 5/10 (2013.01); A61K 2800/4324 (2013.01); A61K 2800/882 (2013.01)] | 20 Claims |
|
1. A method of dyeing keratin fibers, comprising applying to the keratin fibers:
(a) at least one direct dye; and
(b) at least one compound of formula (A):
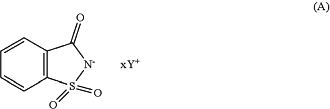 wherein:
Y+, which can be the same or different, represents a cationic counterion chosen from compounds of formula (Ia), (Ib), (Ic), and/or (Id) below:
 wherein in formula (Ia), R1, R2, R3 and R4 are independently chosen from:
a hydrogen atom; and/or
a linear or branched, saturated or unsaturated, C1-C12 hydrocarbon-based group, optionally: (i) substituted with one or more groups chosen from hydroxyl, thiol, (C1-C6)alkylthio, amino, —NH3+, (C1-C6)dialkylamino, (C1-C6)alkylamino, (C1-C4)alkoxyl, carboxyl, carboxylate, —SO3H, —SO3−, —C(O)NH2, —NHC(NH)—NH2, —C(O)ORa, —OC(O)—Ra, —C(O)NHRa, —NH—C(O)—Ra, phenyl, indolyl, phenol, furan, guaiacol, imidazole, or guanine; or (ii) interrupted by one or more group(s) chosen from —C(O)—, —NRb—, —C(NRb)—, —OC(O)—, —C(O)O—, —C(O)NRb— or —NRbC(O)—, with:
Ra representing a (C1-C4)alkyl group; and
Rb representing a hydrogen atom or a (C1-C4)alkyl group;
wherein the overall charge of the compounds of formula (Ia) is positive;
 wherein in formula (Ib):
X represents an oxygen atom or an NRb group, Rb representing a hydrogen atom or a (C1-C4)alkyl group;
R5, R6 and R7 represent independently of one another:
a hydrogen atom;
a carboxyl or carboxylate group;
a —C(O)NH2 group; and/or
a linear or branched, saturated or unsaturated, C1-C12 hydrocarbon-based group, optionally: (i) substituted with one or more groups chosen from hydroxyl, thiol, (C1-C6)alkylthio, amino, —NH3+, (C1-C6)dialkylamino, (C1-C6)alkylamino, (C1-C4)alkoxyl, carboxyl, carboxylate, —SO3H, —SO3−, —C(O)NH2, —NHC(NH)—NH2, —C(O)ORa, —OC(O)—Ra, —C(O)NHRa, —NH—C(O)—Ra, phenyl, indolyl, phenol, furan, guaiacol, imidazole, or guanine; or (ii) interrupted by one or more group(s) chosen from —C(O)—, —NRb—, —C(NRb)—, —OC(O)—, —C(O)O—, —C(O)NRb— or —NRbC(O)—, with:
Ra representing a (C1-C4)alkyl group; and
Rb representing a hydrogen atom or a (C1-C4)alkyl group;
wherein the overall charge of the compounds of formula (Ib) is positive;
 wherein in formula (Ic):
Het represents a cationic saturated heterocyclic group comprising:
from 5 to 10 ring members; and
in addition to the ammonium bearing R′1 and R′2, optionally also bearing one or two atoms chosen from nitrogen and/or oxygen atoms;
the heterocyclic group being optionally substituted with one or more groups R′3, which is independently chosen from a hydroxyl group, an amino group, a —C(O)OR′4 group, an —OC(O)—R′4 group, a —C(O)NHR′4 group, an —NH—C(O)—R′4 group, a linear or branched, saturated or unsaturated, C1-C12 hydrocarbon-based group, optionally substituted with one or more groups chosen from: hydroxyl, amino, (C1-C6)dialkylamino, (C1-C6)alkylamino, carboxyl, carboxylate, carbamide, (C1-C4)alkoxyl, —SO3H, —SO3− or phenyl;
R′4 representing a hydrogen atom, a linear or branched, saturated or unsaturated, C1-C12 hydrocarbon-based group, optionally substituted with one or more groups chosen from hydroxyl, amino, (C1-C6)dialkylamino or (C1-C6)alkylamino; and
R′1 and R′2 are independently chosen from a hydrogen atom or a linear or branched, saturated or unsaturated, C1-C12 hydrocarbon-based group, optionally substituted with one or more groups chosen from hydroxyl, amino, (C1-C6)dialkylamino, (C1-C6)alkylamino, carboxyl, carboxylate, carbamide, (C1-C4)alkoxyl, —SO3H, —SO3−, or phenyl;
wherein the overall charge of the compounds of formula (Ic) is positive;
 wherein in formula (Id):
Het′ represents a cationic aromatic unsaturated heterocyclic group comprising:
from 5 to 10 ring members; and
in addition to the ammonium bearing R″1, optionally also bearing one or two atoms chosen from nitrogen and/or oxygen atoms;
said heterocyclic group being optionally substituted with one or more groups R″2 which is independently chosen from a hydroxyl group, an amino group, or a linear or branched, saturated or unsaturated, C1-C12 hydrocarbon-based group, optionally substituted with one or more group(s) chosen from hydroxyl, amino, (C1-C6)dialkylamino, (C1-C6)alkylamino, carboxyl, carboxylate, carbamide, (C1-C4)alkoxyl, —SO3H, —SO3− or phenyl; and
R″1 represents a linear or branched, saturated or unsaturated, C1-C12 hydrocarbon-based group, optionally substituted with one or more groups chosen from hydroxyl, amino, (C1-C6)dialkylamino, (C1-C6)alkylamino, carboxyl, carboxylate, carbamide, (C1-C4)alkoxyl, —SO3H, —SO3−, or phenyl;
wherein the overall charge of the compounds of formula (Id) is positive; and
x is a stoichiometric coefficient configured to balance the electrical neutrality of the compound of formula (A);
wherein a) and b) are applied simultaneously or sequentially to the keratin fibers.
|