| CPC A61K 38/29 (2013.01) [A61K 47/551 (2017.08); A61K 47/60 (2017.08)] | 12 Claims |
|
1. A pharmaceutical composition comprising one or more parathyroid hormone 1-34 (PTH(1-34)) peptide(s) conjugated via a poly(ethylene glycol) (PEG) scaffold to a non-hormonal vitamin D moiety at the carbon 3 position (PTH conjugate); wherein the scaffold is not conjugated to an amine group of the PTH(1-34); wherein the PEG scaffold consist of 36 subunits at a molecular weight of 1,584 Daltons; wherein the PTH conjugate consists of the acetate salt of
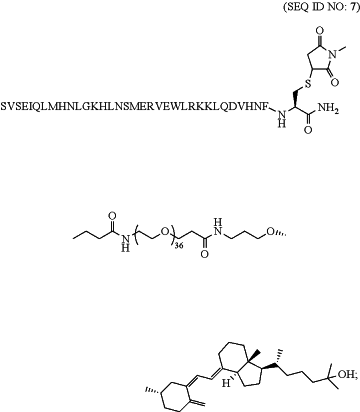 and wherein the composition comprises a pharmaceutically acceptable excipient.
|