| CPC A61K 31/519 (2013.01) [A61K 9/2009 (2013.01); A61K 9/2013 (2013.01); A61K 9/2018 (2013.01); A61K 9/2054 (2013.01); A61K 9/2866 (2013.01)] | 23 Claims |
|
1. A solid tablet for oral administration comprising:
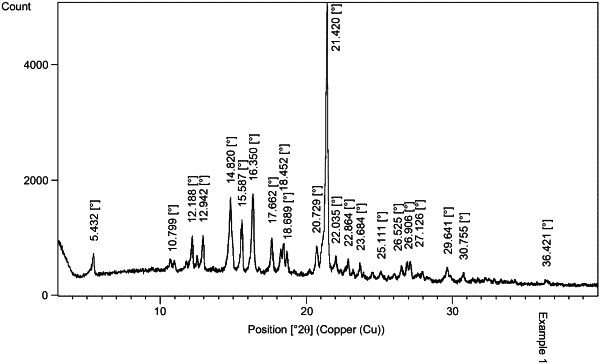
80 mg, 160 mg, or 320 mg micronized zanubrutinib in crystal form A;
a filler comprising lactose in an amount of about 20% to about 70% by mass of the solid tablet; and
a glidant in an amount from about 0.1% to about 20% by mass of the solid tablet, wherein the glidant is powdered cellulose, magnesium trisilicate, colloidal silica, talc powder, or any combination thereof,
provided that if the solid tablet contains lactose as the only filler in the solid tablet, the amount of lactose in the solid tablet is not less than that of zanubrutinib,
wherein at least about 80% of zanubrutinib is dissolved within 60 minutes in a solution having a pH value of about 1.2 and comprising sodium lauryl sulfate in an amount of about 0.5% by mass of the solution at a temperature of 37±0.5° C. with a rotating speed of 100 rpm.
|