| CPC A61K 31/506 (2013.01) [A61K 31/18 (2013.01); A61K 31/277 (2013.01); A61K 31/341 (2013.01); A61K 31/381 (2013.01); A61K 31/40 (2013.01); A61K 31/415 (2013.01); A61K 31/4164 (2013.01); A61K 31/437 (2013.01); A61K 31/4418 (2013.01); A61K 31/4436 (2013.01); A61K 31/4439 (2013.01); A61K 31/496 (2013.01); A61K 31/497 (2013.01); A61K 31/4985 (2013.01); A61K 31/5377 (2013.01); A61K 31/675 (2013.01); A61P 35/00 (2018.01); C07C 311/08 (2013.01); C07D 207/34 (2013.01); C07D 213/56 (2013.01); C07D 231/12 (2013.01); C07D 231/14 (2013.01); C07D 233/90 (2013.01); C07D 307/68 (2013.01); C07D 333/38 (2013.01); C07D 333/70 (2013.01); C07D 401/04 (2013.01); C07D 401/14 (2013.01); C07D 403/04 (2013.01); C07D 409/04 (2013.01); C07D 409/14 (2013.01); C07D 413/14 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07F 9/65583 (2013.01)] | 16 Claims |
|
1. A compound represented by Formula (IVB):
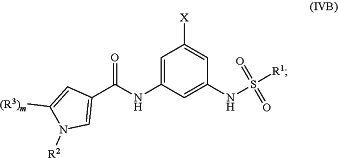 or a pharmaceutically acceptable salt thereof, wherein:
m is 1;
R1 is —CH3;
X is Cl;
R2 is —CH3;
R3 is pyrimidinyl or pyridinyl, each of which is optionally substituted with 1 or 2 R4;
R4 is halo, —OC1-3 alkyl, or 4- to 6-membered monocyclic heterocyclyl, wherein the 4- to 6-membered monocyclic heterocyclyl is optionally substituted with 1 or 2 halo or C1-3haloalkyl.
|