| CPC C07D 487/04 (2013.01) [C07B 2200/13 (2013.01)] | 28 Claims |
|
1. A solid form of tabernanthalog monofumarate salt of the following formula:
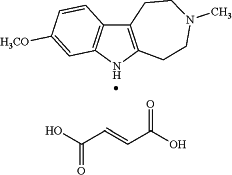 wherein the solid form is crystalline polymorphic Pattern #6a;
(i) wherein crystalline polymorphic Pattern #6a is characterized by an X-ray powder diffraction diffractogram comprising two signals at angles (°2θ) selected from the group consisting of 19.6°±0.2 °2θ, 20.8°±0.2 °2θ, and 26.2°±0.2 °2θ; or
(ii) wherein crystalline polymorphic Pattern #6a is characterized by an X-ray powder diffraction diffractogram comprising three signals at angles (°2θ) selected from the group consisting of 16.2°±0.2 °2θ, 18.9°±0.2 °2θ, 20.2°±0.2 °2θ, and 24.9°±0.2 °2θ; and
wherein the X-ray powder diffraction diffractogram signals are present when the X-ray powder diffraction is carried out using Cu Kα1 radiation.
|
|
8. A pharmaceutical composition comprising a pharmaceutically acceptable excipient and the solid form of claim 1.
|