| CPC C02F 3/006 (2013.01) [C02F 3/305 (2013.01); C02F 2209/06 (2013.01); C02F 2209/08 (2013.01); C02F 2209/10 (2013.01); C02F 2209/16 (2013.01); C02F 2209/22 (2013.01); C02F 2209/44 (2013.01)] | 8 Claims |
|
1. A method, comprising:
S1: establishing a model 1 and a model 2 for simultaneous simulation of microbial dissolved organic nitrogen (mDON) and inorganic nitrogen (DIN) in denitrification processes; and
S2: selecting the model 1 or the model 2 according to a set carbon/nitrogen ratio to collaboratively optimize concentration values of mDON and DIN in an effluent of a denitrification process, to obtain best process operation parameter values;
wherein:
in S1, operations for establishing the models 1 and 2 comprise:
S1-1: data collection: measuring chemical oxygen demand (COD), total nitrogen (TN), inorganic nitrogen (iDIN), dissolved organic nitrogen (rDON) and pH in an influent of a target sewage plant in the denitrification process, inorganic nitrogen (eDIN) and dissolved organic nitrogen (eDON) in the effluent, dissolved oxygen (DO), a hydraulic retention time (t) of a denitrification stage, and a mixed liquor suspended solid (MLSS) of activated sludge;
S1-2: model construction: according to a kinetic process of production, transformation and consumption of mDON during a complete denitrification process, adding mDON as a new component and a carbon/nitrogen ratio as a new parameter into an activated sludge model (ASM), and constructing the models 1 and 2 at different carbon/nitrogen ratios by using mDON and DIN as objects;
S1-3: model initialization: initializing the models 1 and 2 based on data collected in S1-1, calculated values of model parameters and the models 1 and 2 constructed in S1-2;
S1-4: model calibration: calibrating a parameter estimation function based on simulated mDON and DIN kinetics and a result of sensitivity analysis; and
S1-5: model establishment: replacing initial parameter values in the models 1 and 2 with parameter calibration values to obtain calibrated models 1 and 2;
values of 10 components and 9 processes are modeled in the model 1 by using 22 parameters and a kinetic parameter of an inhibition constant (KIAS) of the anoxic substrate of heterotrophic bacteria; and the values of the 10 components and the 9 processes are modeled in the model 2 by using the 22 parameters; wherein:
the 10 components comprise: heterotrophic bacteria XH, particulate inert substance XI, dissolved biodegradable organic matter SS, microbial organic nitrogen SmDON, ammonia nitrogen SNH, nitrate nitrogen SNO3, nitrite nitrogen SNO2, nitric oxide SNO, nitrous oxide SN2O and alkalinity SALK;
the 9 processes comprise: four-step anoxic growth of heterotrophic bacteria based on the dissolved biodegradable organic matter, comprising conversion of nitrate nitrogen into nitrite nitrogen, conversion of nitrite nitrogen into nitric oxide, conversion of nitric oxide into nitrous oxide and conversion of nitrous oxide into nitrogen, and decay of heterotrophic bacteria, ammonification of microbial dissolved organic nitrogen, assimilative reduction of nitrate nitrogen into nitrite nitrogen and assimilative reduction of nitrite nitrogen into ammonia nitrogen; and
the 22 parameters comprise: a yield coefficient YH of anoxic SS-based growth of heterotrophic bacteria, an oxygen containing proportion iXB of organism, a proportion fH,DON of mDON formed by heterotrophic bacteria based on organism growth, a proportion f1 of inert substances produced by organism, a maximum specific growth rate μH of anoxic growth of heterotrophic bacteria, a half-saturation utilization constant KS of a substrate of heterotrophic bacteria, an ammonia half-saturation constant KH,NH of heterotrophic bacteria, an anoxic growth factor η2 of heterotrophic bacteria in a process 2, the anoxic growth factor η3 of heterotrophic bacteria in a process 3, the anoxic growth factor η4 of heterotrophic bacteria in a process 4, the nitrate nitrogen half-saturation constant KNO3, a nitrite nitrogen half-saturation constant KNO2, a nitric oxide half-saturation constant KNO, a nitrous oxide half-saturation constant KN2O, a decay coefficient bH of heterotrophic bacteria, an ammoniated mDON half-saturation constant KH,DON of heterotrophic bacteria, an ammonification rate κa of microbial dissolved organic nitrogen, a NO3−-N half-saturation constant K7,NO3 of ANRA, an inhibition constant K17NH of ammonia nitrogen in the ANRA process, an inhibition constant K18NO2 of nitrite nitrogen in the ANRA process, a half-saturation constant K8,NO2 of nitrite nitrogen in the ANRA process and an oxygen half-saturation constant KH,O of heterotrophic bacteria;
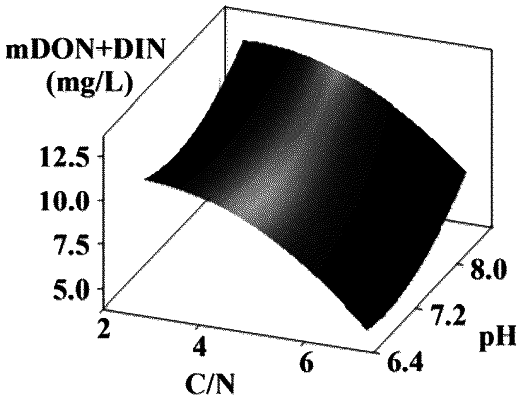
the models 1 and 2 are divided according to the carbon/nitrogen ratio in the influent:
(1) when the carbon/nitrogen ratio is less than or equal to 4, the model 1 is selected, and the kinetic equations for the model 1 are as follows:
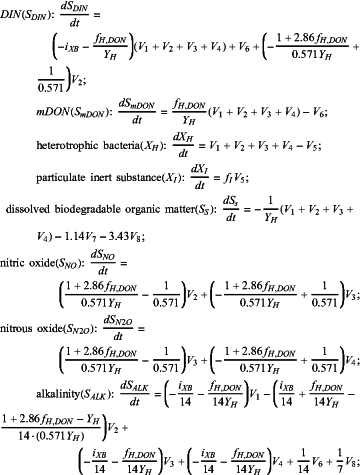 (2) when the carbon/nitrogen ratio is greater than 4, the model 2 is selected, and the kinetic equations for the model 2 are as follows:
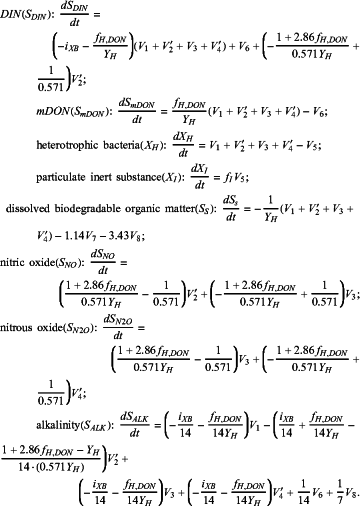 |