| CPC B01J 19/0006 (2013.01) [A61K 51/0491 (2013.01); B01J 19/004 (2013.01); B65B 1/00 (2013.01); B65B 3/003 (2013.01); G21G 1/10 (2013.01); A61J 3/002 (2013.01); B01J 19/0046 (2013.01); B01J 2219/00049 (2013.01); B01J 2219/0072 (2013.01); B01J 2219/00351 (2013.01); B01J 2219/00355 (2013.01); B01J 2219/00585 (2013.01); B01J 2219/00693 (2013.01); B01J 2219/00702 (2013.01); B01J 2219/00722 (2013.01); B01J 2219/00759 (2013.01); G21G 2001/0015 (2013.01)] | 20 Claims |
|
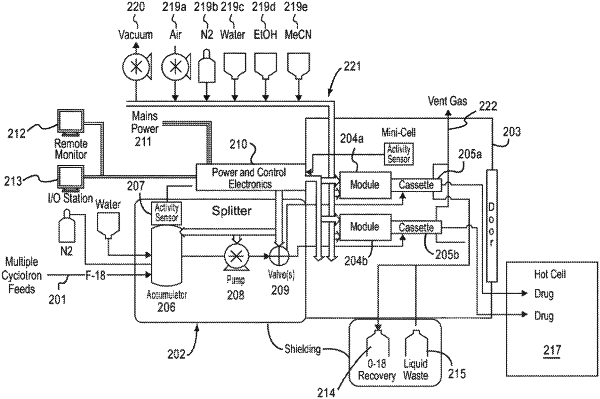
1. A radiopharmaceutical production system comprising:
a cyclotron;
a synthesis unit operatively connected to the cyclotron and to a reagent pack, the synthesis unit comprising at least one modular, disposable cassette unit for receiving a radionuclide from the cyclotron and one or more pharmaceutical reagents from the reagent pack;
a vial filler operatively connected to the synthesis unit for dispensing a radiopharmaceutical produced by the synthesis unit into a vial;
a quality control unit operatively connected to the synthesis unit for performing automated quality control on a sample of at least one dose of the radiopharmaceutical; and
a control platform operatively connected to at least one of the synthesis unit and the quality control unit for receiving therefrom and for associating a unique identifier with each dose of the radiopharmaceutical synthesized by the system.
|