| CPC A61K 31/553 (2013.01) [A61K 31/282 (2013.01); A61K 31/4184 (2013.01); A61K 31/4745 (2013.01); A61K 31/4985 (2013.01); A61K 31/519 (2013.01); A61K 31/52 (2013.01); A61K 31/635 (2013.01); A61K 31/7048 (2013.01); A61K 33/243 (2019.01); A61K 35/17 (2013.01); A61K 38/07 (2013.01); A61K 39/3955 (2013.01); A61K 47/6849 (2017.08); A61P 35/02 (2018.01); C07D 471/14 (2013.01); C07D 491/147 (2013.01); C07D 498/14 (2013.01); C07D 498/22 (2013.01); C07D 513/14 (2013.01); C07D 515/14 (2013.01); C07D 519/00 (2013.01)] | 28 Claims |
|
1. A compound of Formula (A), or an N-oxide thereof, or a pharmaceutically acceptable salt, solvate, polymorph, tautomer, stereoisomer, or an isotopic form of said compound of Formula (A) or N-oxide thereof:
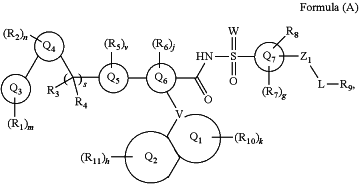 wherein
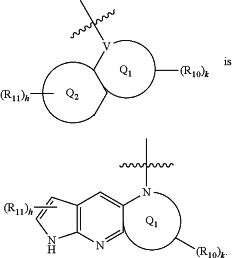 wherein
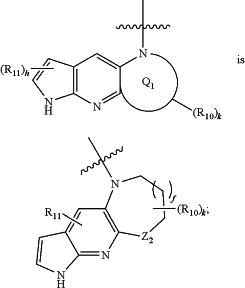 Q3 is aryl or heteroaryl;
Q4 is cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl;
Q5 is 6-membered heterocycloalkyl, or 6-membered heterocycloalkenyl;
Q6 is 6-membered aryl, or 6 membered heteroaryl;
Q7 is 6-membered aryl or 6 membered heteroaryl, each of which is optionally fused with a benzene, heteroarene, cycloalkane, cycloalkene, heterocycloalkane, or heterocycloalkene;
each of R1, R2, R3, R4, R5, R6, R7, R8, and R10, independently, is H, D, alkyl, spiroalkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, heterocycloalkyl, spiroheterocycloalkyl, heterocycloalkenyl, aryl, heteroaryl, halo, nitro, oxo, cyano, ORa, SRa, alkyl-Ra, NH(CH2)pRa, C(O)Ra, S(O)Ra, SO2Ra, C(O)ORa, OC(O)Ra, NRbRc, C(O)N(Rb)Rc, N(Rb)C(O)Rc, —P(O)RbRc, -alkyl-P(O)RbRc, —S(O)(═N(Rb))Rc, —N═S(O)RbRc, ═NRb, SO2N(Rb)Rc, or N(Rb)SO2Rc, in which said cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl is optionally substituted with one or more Rd;
R9 is R9A or R9B;
R9A is H, D, alkyl, spiroalkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, heterocycloalkyl, spiroheterocycloalkyl, heterocycloalkenyl, aryl, heteroaryl, halo, nitro, oxo, cyano, ORa, SRa, alkyl-Ra, NH(CH2)pRa, C(O)Ra, S(O)Ra, SO2Ra, C(O)ORa, OC(O)Ra, NRbRc, C(O)N(Rb)Rc, N(Rb)C(O)Rc, —P(O)RbRc, -alkyl-P(O)RbRc, —S(O)(═N(Rb))Rc, —N═S(O)RbRc, ═NRb, SO2N(Rb)Rc, or N(Rb)SO2Rc, in which said cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl is optionally substituted with one or more Rd;
R9B is a small molecule E3 ubiquitin ligase binding moiety that binds an E3 ubiquitin ligase;
Rd, independently, is H, D, alkyl, spiroalkyl, alkenyl, alkynyl, halo, cyano, amine, nitro, hydroxy, ═O, —P(O)RbbRcc, -alkyl-P(O)RbbRcc, —S(O)(═N(Rbb))Rcc, —N═S(O)RbbRcc, ═NRbb, C(O)NHOH, C(O)OH, C(O)NH2, alkoxy, alkoxyalkyl, haloalkyl, hydroxyalkyl, aminoalkyl, alkylcarbonyl, alkoxycarbonyl, alkylcarbonylamino, alkylamino, oxo, halo-alkylamino, cycloalkyl, cycloalkenyl, heterocycloalkyl, spiroheterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl, in which said alkyl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl of Rd is optionally substituted with one or more Re;
Ra, Rb, Rc, Rbb and Rcc independently, is H, D, alkyl, spiroalkyl, alkenyl, alkynyl, alkoxy, alkoxyalkyl, haloalkyl, hydroxyalkyl, aminoalkyl, cycloalkyl, cycloalkenyl, heterocycloalkyl, spiroheterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl, in which said alkyl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl of Ra, Rb, Rc, Rbb and Rcc is optionally substituted with one or more Re;
Re, independently, is H, D, alkyl, spiroalkyl, alkenyl, alkynyl, halo, cyano, amine, nitro, hydroxy, ═O, C(O)NHOH, alkoxy, alkoxyalkyl, haloalkyl, hydroxyalkyl, aminoalkyl, alkylcarbonyl, alkoxycarbonyl, alkylcarbonylamino, alkylamino, oxo, halo-alkylamino, cycloalkyl, cycloalkenyl, heterocycloalkyl, spiroheterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl;
Z1 is a bond, (CH2)p, N(H), O, S, C(O), S(O)2, OC(O), C(O)O, OSO2, S(O)2O, C(O)S, SC(O), C(O)C(O), C(O)N(H), N(H)C(O), S(O)2NH, NHS(O)2, OC(O)O, OC(O)S, OC(O)N(H), N(H)C(O)O, N(H)C(O)S, N(H)C(O)N(H), (CH2)pN(H)(CH2)q, (CH2)pN(H)C(O)(CH2)q, (CH2)pC(O)N(H)(CH2)q, OC(O)N(H)(CH2)p+1N(H)(CH2)q, a bivalent alkenyl group, or a bivalent alkynyl group;
Z2 is —O—;
L is L1 or L2;
L1 is bond, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl, in which said alkenyl, alkynyl, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl of L1 is optionally substituted with one or more Rd;
L2 is an alkyl in which one or more -Li- are optionally inserted between any two adjacent carbon atoms;
-Li- is —N(Ra)—, —O—, —S—, —C(O)—, —S(O)2—, —OC(O)—, —C(O)O—, —OSO2—, S(O)2O—, —C(O)S—, —SC(O)—, —C(O)C(O)—, —C(O)N(Ra)—, —N(Ra)C(O), —S(O)2N(Ra)—, —N(Ra)S(O)2—, —OC(O)O—, —OC(O)S—, —OC(O)N(Ra)—, —N(Ra)C(O)O—, —N(Ra)C(O)S—, —N(Ra)C(O)N(Ra)—, a bivalent alkenyl group, a bivalent alkynyl group, a bivalent cycloalkyl group, a bivalent heterocycloalkyl group, a bivalent aryl group, a bivalent heteroaryl group;
W is O or N(Ra);
two of R1 group, taken together with the atoms to which they are attached, may optionally form a cycloalkyl or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of R1, is optionally substituted with one or more Rd;
two of R2 group, taken together with the atom(s) to which they are attached, may optionally form a cycloalkyl or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of R2, is optionally substituted with one or more Rd;
R3 and R4 group, taken together with the atom to which they are attached, may optionally form a cycloalkyl or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of R3 and R4, is optionally substituted with one or more Rd;
two of R5 group, taken together with the atom(s) to which they are attached, may optionally form a cycloalkyl or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of R5, is optionally substituted with one or more Rd;
two of R6 group, taken together with the atoms to which they are attached, may optionally form a cycloalkyl or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of R6, is optionally substituted with one or more Rd;
two of R10 group, taken together with the atom(s) to which they are attached, may optionally form a cycloalkyl or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of R10, is optionally substituted with one or more Rd;
R2 and R5 group, taken together with the atoms to which they are attached, may optionally form a cycloalkyl or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of R2 and R5, is optionally substituted with one or more Rd;
R3 and R5 group, taken together with the atoms to which they are attached, may optionally form a cycloalkyl or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of R3 and R5, is optionally substituted with one or more Rd;
Rb and Rc group, taken together with the atom to which they are attached, may optionally form a cycloalkyl, or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of Rb and Re, is optionally substituted with one or more Rc;
two of Rd group, taken together with the atom(s) to which they are attached, may optionally form a cycloalkyl, or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of Rd, is optionally substituted with one or more Re;
two of Re group, taken together with the atom(s) to which they are attached, may optionally form a cycloalkyl or heterocycloalkyl, in which said cycloalkyl or heterocycloalkyl of Re is optionally substituted with one or more groups selected from H, D, alkyl, alkenyl, alkynyl, halo, cyano, amine, nitro, hydroxy, C(O)NHOH, alkoxy, alkoxyalkyl, haloalkyl, hydroxyalkyl, aminoalkyl, alkylcarbonyl, alkoxycarbonyl, alkylcarbonylamino, alkylamino, oxo, halo-alkylamino, cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, aryl, or heteroaryl;
each of g and j is, independently, 0, 1, 2, or 3;
each of n, v and k is, independently, 0, 1, 2, 3, 4, 5, 6, 7, or 8;
s is 0 or 1;
f is 0; and
each of m, p, and q is, independently, 0, 1, 2, 3, 4, or 5.
|