| CPC A61K 31/536 (2013.01) [A61K 47/64 (2017.08); A61K 47/65 (2017.08); A61K 47/6803 (2017.08); A61K 47/6869 (2017.08); A61P 35/00 (2018.01); C07K 16/32 (2013.01); C07K 2317/24 (2013.01)] | 7 Claims |
|
1. A preparation method of an antibody-drug conjugate represented by Formula (I), or a pharmaceutically acceptable salt or stereoisomer thereof, or a solvate of the foregoing,
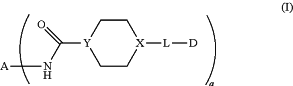 wherein:
A is an anti-ErbB2 antibody, wherein the anti-ErbB2 antibody is Trastuzumab;
X is N;
Y is N or CR1, and R1 is H or C1-C10 alkyl;
L is
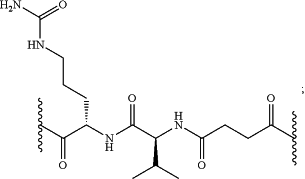 D is a cytotoxic agent group of Formula (D1) or a stereoisomer thereof,
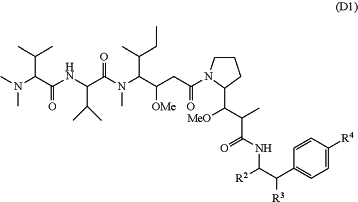 wherein R2 is —CH2N3, R3 is selected from the group consisting of H and —OH, and R4 is selected from the group consisting of H, —NH2, Cl, Br, I and —OS(O)2R6, wherein R6 is H, C1-C8 alkyl, C3-C8 cycloalkyl or C6-C14 aryl, and the alkyl, cycloalkyl and aryl are each optionally substituted with one or more halogen substituents;
a is an integer selected from the group consisting of 2-10; and
all the cytotoxic agent groups are conjugated to the lysine residues of the same peptide segment of the light chain of the antibody;
the method comprising the steps of:
(1) preparing a compound of Formula (I-A):
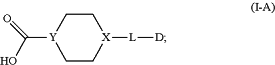 (2) obtaining a compound of Formula (I-B) by reacting the compound of Formula (I-A) with pentafluorophenol using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, N-hydroxysuccinimide and/or dichloromethane
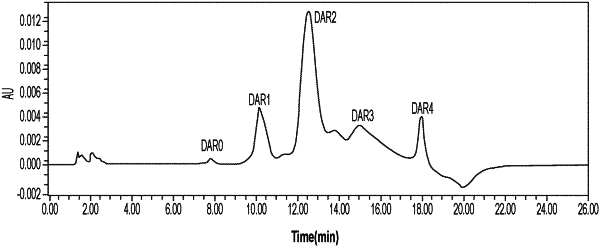
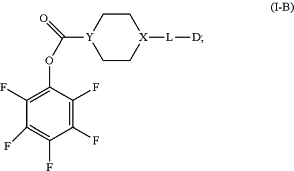 (3) obtaining a mixture of a variety of antibody-drug conjugates having different a values by conjugating the compound of Formula (I-B) from step (2) with the anti-ErbB2 antibody; and
(4) obtaining the antibody-drug conjugate represented by Formula (I) by purifying the mixture from step (3) with hydrophobic interaction chromatography (HIC) method, said method utilizing a stationary phase that comprises a phenyl resin;
wherein a mobile phase A of the HIC method comprises ammonia sulfate and disodium hydrogen phosphate; and a mobile phase B of the HIC method comprises disodium hydrogen phosphate and isopropanol.
|