| CPC A61K 31/513 (2013.01) [A61P 3/04 (2018.01)] | 18 Claims |
|
1. A method of treating a subject suffering from antipsychotic-induced weight gain, the method comprising administering to the subject an effective amount of the cyclohexyl pyrimidine glucocorticoid receptor modulator (GRM) miricorilant, (E)-6-(4-Phenylcyclohexyl)-5-(3-trifluoromethylbenzyl)-1H-pyrimidine-2,4-dione, which has the structure:
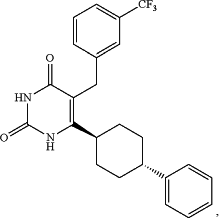 while said subject is administered an antipsychotic medication, wherein said treatment is effective to:
reduce the body weight of a subject, as compared to baseline body weight of said subject prior to said administration of said GRM, wherein the subject has previously been administered an antipsychotic medication; or
reduce the weight gain of a subject over time while taking an antipsychotic medication and said GRM, as compared to average weight gain of subjects taking that antipsychotic medication in the absence of the GRM; or
reduce blood levels of triglycerides as compared to triglyceride levels in the blood of said subject prior to said administration of said GRM, wherein the subject has previously been administered an antipsychotic medication; or
reduce blood levels of the liver enzymes alanine aminotransferase (ALT), separate aminotransferase (AST), or both as compared to baseline liver enzyme levels in the blood of said subject prior to said administration of said GRM, wherein the subject has previously been administered an antipsychotic medication; or
reduce plasma insulin level as compared to the baseline plasma insulin level of said subject prior to said administration of said GRM, wherein the subject has previously been administered an antipsychotic medication; or
reduce insulin resistance as compared to baseline insulin resistance of said subject prior to said administration of said GRM, wherein the subject has previously been administered an antipsychotic medication (as measured by HOMA-IR or HOMA2-IR); or
combinations thereof.
|