| CPC A61K 31/05 (2013.01) [A61K 9/0019 (2013.01); A61P 25/00 (2018.01); H03M 13/1165 (2013.01); H03M 13/255 (2013.01); H03M 13/2707 (2013.01); H03M 13/2778 (2013.01); H03M 13/3761 (2013.01); H03M 13/3769 (2013.01); H03M 13/6356 (2013.01); H03M 13/6362 (2013.01); H04L 1/0041 (2013.01); H04L 1/0045 (2013.01); H04L 1/0057 (2013.01); H04L 1/0065 (2013.01); H04L 1/0067 (2013.01); H04L 1/0071 (2013.01); H03M 13/152 (2013.01); H04L 27/20 (2013.01)] | 18 Claims |
|
1. A method of antagonizing glycoprotein VI receptor, comprising administration of a therapeutically effective amount of the following compound of Formula (I) to a subject:
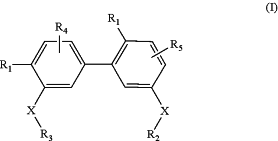 wherein
X is (CH2)1-6;
R1 is phosphate or carbonate;
R2 and R3 are each independently C2-6alkenyl, C1-10alkyl, —O—C1-10alkyl or —NH—C1-10alkyl, wherein the alkyl or alkenyl is unsubstituted or substituted; and
R4 and R5 are each independently one to three H, halogen, —OH, —NH2, NO2, C1-10alkyl, C2-6alkenyl, —O—C1-10alkyl or —NH—C1-10alkyl;
or a pharmaceutically acceptable salt thereof;
wherein the compound or a pharmaceutically acceptable salt thereof is administered by intravenous injection; and
wherein the compound or a pharmaceutically acceptable salt thereof is administered at a dose of about 0.01 to about 1.0 mg per kg body weight.
|