| CPC A61F 2/0095 (2013.01) [A61F 2/00 (2013.01); A61F 2/2418 (2013.01); A61F 2/2427 (2013.01); A61L 2/0088 (2013.01); A61L 2/0094 (2013.01); B65D 81/18 (2013.01); B65D 81/22 (2013.01); A61L 2202/181 (2013.01); A61L 2202/21 (2013.01)] | 10 Claims |
|
1. An assembly including:
an elongated delivery device;
an implant loaded onto a first portion of the elongated delivery device;
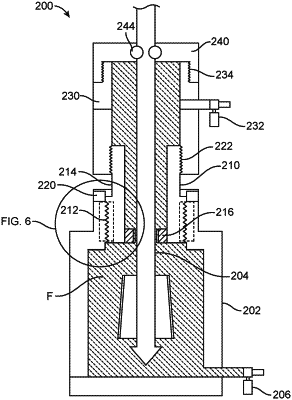
a storage container including a first section and a second section; wherein the implant is positioned within the first section and the first portion of the delivery device is positioned in both the first and second sections;
a first seal positioned to retain sterilization fluid within the first section; and
a second seal positioned between at least a portion of the delivery device and the second section, wherein the first seal is impermeable to liquid and the second seal is gas permeable.
|