| CPC H04L 9/14 (2013.01) [G06N 3/08 (2013.01); G06N 5/025 (2013.01); G06N 20/00 (2019.01)] | 17 Claims |
|
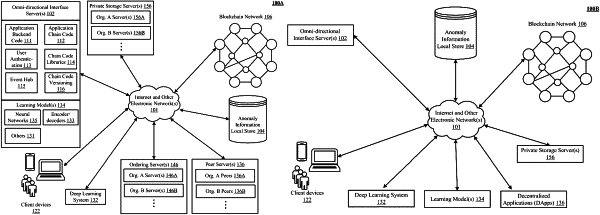
1. A method of securing information associated with a clinical trial, the method including:
storing, to block-level clinical trial structures of a blockchain, an on-chain record origin of clinical trial information received from a first custodian, wherein the on-chain record origin of the clinical trial information is an encrypted version of an original record of the clinical trial information signed with credentials of the first custodian;
receiving, from a first user seeking to validate a veracity of a first off-chain copy of the clinical trial information, a request to view the on-chain record of clinical trial information, from the block-level clinical trial structures stored to the blockchain;
permitting the first user to access the on-chain record of the clinical trial information in dependence upon an authorization;
receiving, from the first user, a validation of the veracity of the first off-chain copy of the clinical trial information as being unaltered from the on-chain record origin, wherein the validation further includes (i) an encrypted version of the first off-chain copy and (ii) credentials of the first user; and
storing, to the blockchain, the validation of the veracity of the first off-chain copy of the clinical trial information.
|