| CPC G16H 20/00 (2018.01) [A61K 35/13 (2013.01); A61K 39/4611 (2023.05); A61K 39/4644 (2023.05); C12N 5/0638 (2013.01); C12N 5/0693 (2013.01); G06Q 10/0832 (2013.01); G16H 10/60 (2018.01); G16H 80/00 (2018.01)] | 19 Claims |
|
1. A system for coordinating manufacturing of a cell therapy product for treating cancer in a patient, the system comprising:
at least one computing device and configured to:
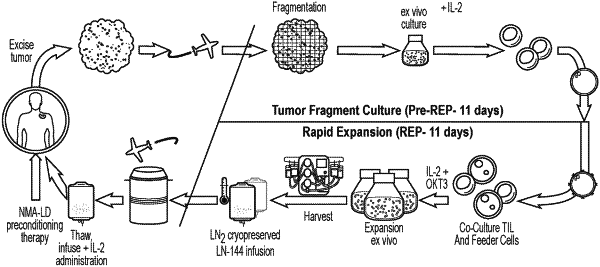
receive and transmit patient information and a cell order request for manufacturing, using a multi-step cell expansion process, the cell therapy product from a population of cells obtained from a tumor resected from the patient, the patient information including a patient-specific identifier, and the cell order request including a cell order identifier, and
determine a schedule for patient treatment events including infusion of the cell therapy product in the patient based on an estimated completion date for obtaining of the cell therapy product manufactured by and received from a manufacturing facility using the multi-step cell expansion process;
generate a container label for a manufacturing container to be used in the multi-step cell expansion process for manufacturing the cell therapy product from at least a portion of the population of cells using a cell expansion technique, the container label comprising information associated with: (a) the cell order request including the cell order identifier, (b) the patient including the patient-specific identifier, and (c) the manufacturing facility where the cell therapy product is to be manufactured using the multi-step cell expansion process, wherein the multi-step cell expansion process comprises:
(i) initiating the multi-step cell expansion process for manufacturing the cell therapy product, and
(ii) initiating quality control assays to determine acceptance parameters at each of a plurality of time points during the multi-step cell expansion process,
receive the acceptance parameters obtained at the plurality of time points during the multi-step cell expansion process;
read the container label at each subsequent time point;
verify a chain of custody and a chain of identity based on the cell order identifier and the patient-specific identifier on the container label at corresponding subsequent time points;
generate a warning signal if:
the patient-specific identifier on the container label for a subsequent manufacturing step does not match the patient-specific identifier on the container label for an immediately preceding manufacturing step; and
determine appropriate further processing steps of the multi-step cell expansion process based on information read from the container label for completing the manufacturing of the cell therapy product.
|