| CPC C07F 9/65583 (2013.01) [C07B 2200/13 (2013.01)] | 14 Claims |
|
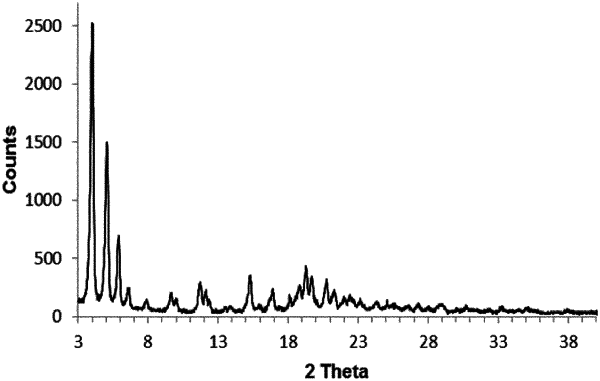
1. A method for the treatment of a disease that is responsive to the inhibition of platelet aggregation, the method comprising administering to a subject in need thereof a pharmaceutical composition comprising as active ingredient a P2Y12 receptor antagonist which is 4-((R)-2-{[6-((S)-3-methoxy-pyrrolidin-1-yl)-2-phenyl-pyrimidine-4-carbonyl]-amino}-3-phosphono-propionyl)-piperazine-1-carboxylic acid butyl ester, or a pharmaceutically acceptable salt thereof, and at least one pharmaceutically acceptable carrier material, wherein 4-((R)-2-{[6-((S)-3-methoxy-pyrrolidin-1-yl)-2-phenyl-pyrimidine-4-carbonyl]-amino}-3-phosphono-propionyl)-piperazine-1-carboxylic acid butyl ester, or the pharmaceutically acceptable salt thereof, is obtained from a crystalline form of 4-((R)-2-{[6-((S)-3-methoxy-pyrrolidin-1-yl)-2-phenyl-pyrimidine-4-carbonyl]-amino}-3-phosphono-propionyl)-piperazine-1-carboxylic acid butyl ester hydrochloride, wherein the crystalline form of 4-((R)-2-{[6-((S)-3-methoxy-pyrrolidin-1-yl)-2-phenyl-pyrimidine-4-carbonyl]-amino}-3-phosphono-propionyl)-piperazine-1-carboxylic acid butyl ester hydrochloride is characterized by:
a. the presence of peaks in the X-ray powder diffraction diagram at the following angles of refraction 2θ: 4.0°, 5.0°, and 15.3°; or
b. the presence of peaks in the X-ray powder diffraction diagram at the following angles of refraction 2θ: 5.2°, 6.8°, and 10.3°; or
c. the presence of peaks in the X-ray powder diffraction diagram at the following angles of refraction 2θ: 5.5°, 11.0°, and 16.6°.
|