| CPC C07D 471/04 (2013.01) [A61P 35/00 (2018.01)] | 15 Claims |
|
1. A compound of formula (I)
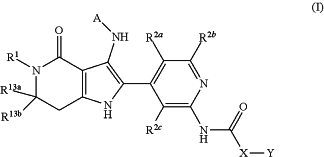 wherein:
R1 represents hydrogen or a group selected from C1-C4-alkyl, C3-C6-cycloalkyl, C3-C6-cycloalkyl-C1-C4-alkyl-, C1-C3-alkoxy-C1-C3-alkyl-, and C1-C5-hydroxyalkyl, wherein said groups are each independently optionally substituted, one or more times, with halogen;
R2a represents hydrogen, hydroxy, halogen, cyano, —NH2, —NHMe, —NMe2, C1-C2-alkoxy, C1-C2-haloalkyl, or C1-C2-haloalkoxy;
R2b represents hydrogen, hydroxy, halogen, cyano, —NH2, —NHR9, or —NMe2;
R2c represents hydrogen, halogen, cyano, —NMe2, C1-C2-alkoxy, or C1-C2-haloalkoxy;
X represents methylene or ethylene,
wherein said methylene and ethylene groups are each independently optionally substituted, one or more times, with R3;
Y represents phenyl or a heteroaryl group,
wherein said phenyl and heteroaryl groups are each independently optionally substituted, one or more times, with R4;
R3 represents hydroxy, halogen, cyano, C1-C3-alkyl, C1-C3-alkoxy, C1-C3-haloalkyl, C1-C2-haloalkoxy, C3-C4-cycloalkyl, —NHR6, R5O—C1-C3-alkyl, R6R7N—C1-C5-alkyl, or R6R7N—X′—C(R10)(R11)—C1-C2-alkyl-;
X′ represents methylene or ethylene;
R4 represents hydroxy, halogen, cyano, C1-C4-alkyl, C1-C4-alkoxy, C1-C4-haloalkyl, C1-C4-haloalkoxy, —NMe2, —CO2R14, —CONR15R16, or —SO2Me;
R5 represents hydrogen, C1-C2-alkyl, C1-C2-haloalkyl, or C3-C4-cycloalkyl;
R6 represents hydrogen, C1-C2-alkyl, C1-C2-haloalkyl, or C3-C4-cycloalkyl and
R7 represents hydrogen, C1-C2-alkyl, C1-C2-haloalkyl, C3-C4-cycloalkyl, or —CO2-C1-C4-alkyl, or
R6 and R7, together with the nitrogen atom to which they are attached, represent a 4- to 7-membered heterocyclic ring, wherein said heterocyclic ring optionally contains one or more further heteroatoms selected from N, O, S, C(═O), S(═O), and SO2, and is independently optionally substituted, one or more times, with halogen, C1-C3-alkyl, C3-cycloalkyl-C1-C2-alkyl-, —NH2, —NHR9, —NR9R9a, or hydroxy;
A represents a group selected from:
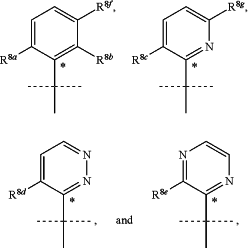 wherein * indicates the point of attachment of said group to the —NH— of formula (I);
R8a represents hydrogen, hydroxy, halogen, cyano, C1-C2-alkyl, or C1-C2-haloalkyl;
R8b represents hydrogen, hydroxy, halogen, cyano, C1-C2-alkyl, or C1-C2-haloalkyl;
R8c represents hydrogen, hydroxy, halogen, cyano, C1-C2-alkyl, or C1-C2-haloalkyl;
R8d represents hydrogen, hydroxy, halogen, cyano, C1-C2-alkyl, or C1-C2-haloalkyl;
R8e represents hydrogen, hydroxy, halogen, cyano, C1-C2-alkyl, or C1-C2-haloalkyl;
R8f represents hydrogen, hydroxy, halogen, cyano, C1-C2-alkyl, or C1-C2-haloalkyl;
R8g represents hydrogen or halogen;
R9 represents C1-C3-alkyl or cyclopropyl;
R9a represents C1-C3-alkyl or cyclopropyl;
R10 represents methyl, halogen, or hydroxy;
R11 represents methyl, halogen, or hydroxy;
R13a represents hydrogen, or a group selected from C1-C4-alkyl, C1-C4-haloalkyl, C3-C4-cycloalkyl, C3-C6-cycloalkyl-C1-C4-alkyl-, and C1-C3-alkoxy-C1-C3-alkyl-, wherein said groups are each independently optionally substituted, one or more times, with halogen and
R13b represents hydrogen, or a group selected from C1-C4-alkyl, C3-C4-cycloalkyl, C3-C6-cycloalkyl-C1-C4-alkyl-, and C1-C3-alkoxy-C1-C3-alkyl-, wherein said groups are each independently optionally substituted, one or more times, with halogen, or,
R13a and R13b, together with the carbon atom to which they are attached, form a C3-C5-cycloalkyl ring or a 4- to 5-membered heterocyclic ring;
R14 represents hydrogen, methyl, or ethyl; and
R15 represents hydrogen, methyl, or ethyl and
R16 represents hydrogen, methyl, ethyl, methoxyethyl, or dimethylaminoethyl, or
R15 and R16, together with the nitrogen atom to which they are attached, represent a 5- to 6-membered heterocyclic ring, wherein said heterocyclic ring optionally contains one or two further heteroatoms selected from N, O, S, C(═O), S(═O), and SO2;
or an N-oxide, a salt, a tautomer, or a stereoisomer of said compound, or a salt of said N-oxide, tautomer, or stereoisomer.
|
|
15. An intermediate compound of general formula (1-3), or a salt thereof:
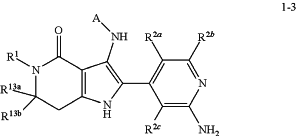 wherein R1, R2a, R2b, R2c, R13a, R13b, and A are as defined for the compound of general formula (I) according to claim 1, with the proviso that R1 is not hydrogen or methyl.
|