| CPC A61B 18/02 (2013.01) [A61B 5/6853 (2013.01); A61B 2018/00077 (2013.01); A61B 2018/00839 (2013.01); A61B 2018/0212 (2013.01)] | 17 Claims |
|
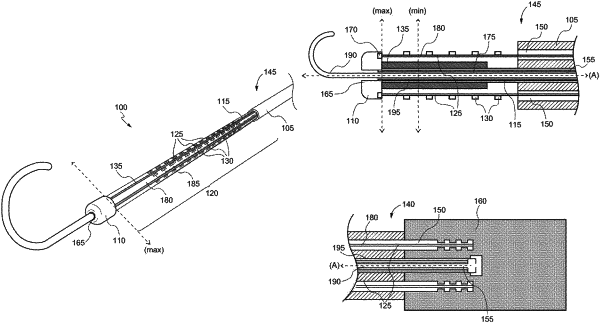
1. A medical device comprising:
a hollow core;
a balloon disposed over at least a portion of the hollow core;
a flexible framework comprising one or more thin film elements formed on at least a portion of the balloon, wherein the one or more thin film elements comprise a plurality of mapping electrodes;
an end cap;
a sheath, wherein the hollow core extends between the end cap and the sheath;
a travel limiter disposed between the one or more thin film elements and the hollow core; and
a pull cable disposed within the sheath and attached to the end cap or the hollow core for retracting the hollow core and anchoring the travel limiter to a distal end of the sheath.
|