| CPC H01M 8/188 (2013.01) [H01M 4/96 (2013.01); H01M 8/0258 (2013.01); H01M 8/04186 (2013.01); H01M 8/04216 (2013.01)] | 19 Claims |
|
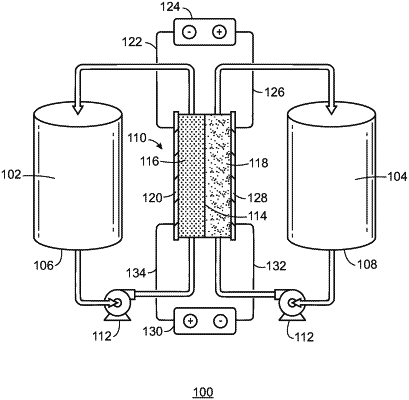
1. A flow cell battery, comprising:
an electrochemical cell, wherein the electrochemical cell comprises:
an ion exchange membrane;
an anode current collector;
a cathode current collector, wherein a space between the ion exchange membrane and the anode current collector forms a first channel, a space between the ion exchange membrane and the cathode current collector forms a second channel, and wherein the ion exchange membrane is configured to allow ions to pass between the first channel and the second channel;
a first tank configured to flow an anolyte through the first channel at a first rate; and
a second tank configured to flow a catholyte through the second channel at a second rate, wherein the catholyte comprises 1.25 molar (M) V, 1.25 M Fe, 6.6 M Cl−, and 0.8 M SO42−.
|