| CPC H01M 4/62 (2013.01) [C01G 33/00 (2013.01); H01M 4/366 (2013.01); H01M 10/0525 (2013.01); H01M 4/505 (2013.01); H01M 4/525 (2013.01); H01M 2004/028 (2013.01)] | 1 Claim |
|
1. A method for producing an all-solid-state battery,
the method comprising:
preparing a solution containing niobium ions and lithium ions,
drying the solution to obtain a lithium niobate precursor, and
obtaining a lithium niobate by heating the lithium niobate precursor at a temperature of from 250° C. to 300° C. for a heating time of more than 0 minute and 10 minutes or less, and
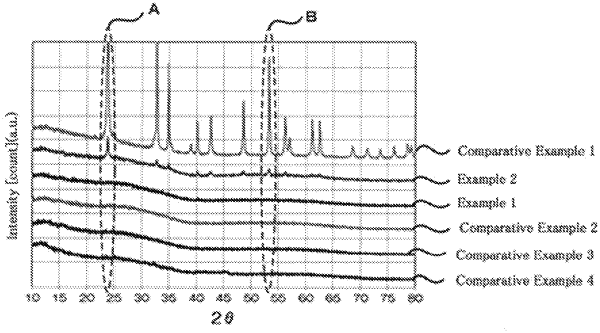
wherein an A/B ratio value of the lithium niobate between a first peak intensity value A at a diffraction angle (2θ) of 23.7°±0.5° and a second peak intensity value B at a diffraction angle (2θ) of 53.2°±0.5°, is 1.96 or more and 2.54 or less, and the peak intensities are observed by X-ray diffraction (XRD) measurement using CuKα radiation;
wherein the all-solid-state battery comprises a cathode comprising a cathode layer, an anode comprising an anode layer, and a solid electrolyte layer disposed between the cathode layer and the anode layer;
wherein the cathode layer contains composite active material particles;
wherein the composite active material particles are composed of cathode active material particles and a covering layer;
wherein the covering layer contains the lithium niobate and covering at least part of the surface of the cathode active material particles;
wherein the solid electrolyte layer contains a sulfide-based solid electrolyte; and
wherein the sulfide-based solid electrolyte contains 10LiI-15LiBr-37.5Li3PS4 as a raw material composition.
|