| CPC G06F 40/166 (2020.01) [G06V 30/10 (2022.01); G10L 15/08 (2013.01); G16H 10/20 (2018.01); G16H 40/20 (2018.01)] | 9 Claims |
|
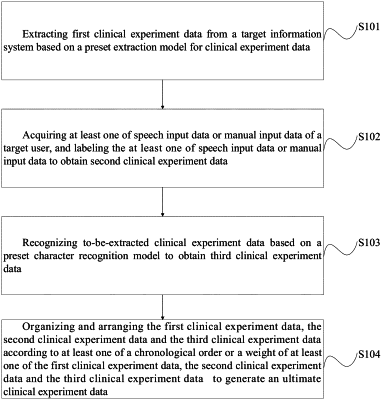
1. An acquisition method for clinical experimental data, comprising:
extracting first clinical experimental data from a target information system based on a preset extraction model for clinical experimental data;
acquiring at least one of speech input data or manual input data of a target user, and labeling the at least one of speech input data or manual input data to obtain second clinical experimental data;
recognizing to-be-extracted clinical experimental data based on a preset character recognition model, to obtain third clinical experimental data; and
organizing and arranging the first clinical experimental data, the second clinical experimental data and the third clinical experimental data according to at least one of a chronological order or a weight of at least one of the first clinical experimental data, the second clinical experimental data and the third clinical experimental data to generate ultimate clinical experimental data;
wherein before the recognizing to-be-extracted clinical experimental data based on a preset character recognition model to obtain third clinical experimental data, the acquisition method for clinical experimental data further comprises: acquiring at least one of a to-be-recognized text or a to-be-recognized image; recognizing characters and a modifying sign based on a preset floating distance threshold to recognize the at least one of the to-be-recognized text or the to-be-recognized image to thus obtain recognized characters, concatenating the recognized characters based on a preset principle to obtain a recognized text; correcting the recognized text based on a preset comparative document to obtain a corrected recognized text, and training a model based on the corrected recognized text to obtain the preset character recognition model;
wherein the preset principle is a nearby principle or a modifying-sign-based principle, and the preset floating distance threshold is a vertical floating distance of a character from a centerline in a vertical direction; and
wherein in a situation that a correction is made due to an omission in writing, the modifying sign is detected; in a situation that vertical distances of characters within the modifying sign relative to the centerline in the vertical direction fluctuate, the characters within the modifying sign are compared with the preset comparative document and a semantic check is performed; and in a situation that a sentence is unclear due to lack of the characters within the modifying sign, the characters within the modifying sign are included into the sentence.
|