| CPC C09K 11/06 (2013.01) [C09K 11/02 (2013.01); H01L 33/502 (2013.01); C09K 2211/1007 (2013.01); C09K 2211/1018 (2013.01); H01L 2933/0091 (2013.01)] | 19 Claims |
|
1. A yellow light emitting device, comprising:
(i) a light source comprising a blue light emitting diode with a center wavelength of emission in a range of from 400 nm to 480 nm, and/or a white light emitting diode having a correlated color temperature between in a range of from 2 700 to 30 000 K;
(ii) a color converter comprising an organic fluorescent dye in a polymer matrix, the organic fluorescent dye comprising a structural unit of formula (I)
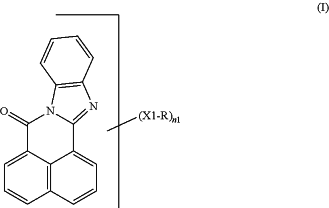 wherein one or more CH groups of the six-membered ring of the benzimidazole structure are optionally replaced by nitrogen and wherein
n1 is a number from 0 to (10-p1) for each structural unit of the formula (1), p1 being a number of the CH groups replaced by nitrogen in the six-membered ring of the benzimidazole structure,
X1 is a chemical bond, O, S, SO, SO2, or NR1, and
R is an aliphatic radical, cycloaliphatic radical, aryl, or heteroaryl, or an aromatic or heteroaromatic ring or ring system, fused to other aromatic rings of the structural unit of the formula (I), or F, Cl, Br, CN, or H when X1 is not a chemical bond, two R radicals optionally being joined to give one cyclic radical,
X1 and R, when n1>one, optionally being the same or different;
R1 is independently hydrogen, C1-C18-alkyl or cycloalkyl, the carbon chain of which optionally comprising an —O—, —S—, —CO—, —SO— and/or —SO2— moiety, or aryl or heteroaryl
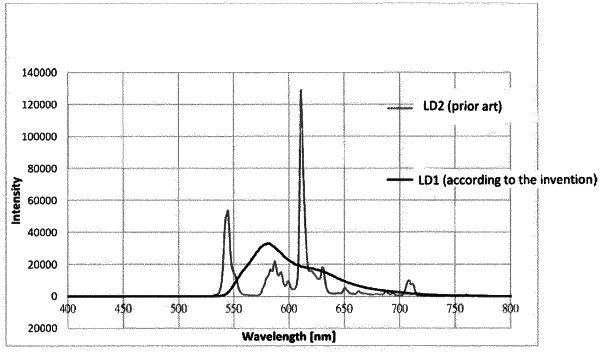
wherein the organic fluorescent dye absorbs at least a part of light emitted by the light source and emits light comprising a wavelength in a range of from 520 to 590 nm,
wherein the organic fluorescent dye in the color converter has a weight percentage satisfying equation (A)
20[%×μm]≤w1×d≤100[%×μm] (A),
wherein w1 is the weight percentage of the organic fluorescent dye in the polymer matrix, based on total polymer weight, and d is thickness of the color converter in μm,
wherein at most 1% of total emitted radiant power of the yellow light emitting device is emitted in a wavelength range shorter than 520 nm, and
wherein the yellow-light emitting device has a luminous efficiency of at least 80 lumen/watt.
|