|
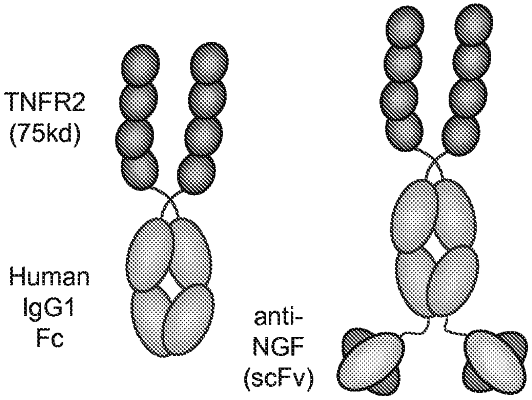
1. A method for controlling or treating pain in a subject, comprising administering to a subject in need thereof an effective amount of a binding molecule comprising an NGF antagonist domain and a TNFα antagonist domain, wherein the NGF antagonist is an anti-NGF antibody, or antigen-binding fragment thereof; wherein the TNFα antagonist domain comprises a soluble, TNFα-binding fragment of TNFR-2; wherein the anti-NGF antibody or fragment thereof comprises a VH domain comprising a set of CDRs HCDR1, HCDR2, HCDR3 and a VL domain comprising a set of CDRs LCDR1, LCDR2 and LCDR3, wherein the HCDR1 comprises the amino acid sequence of SEQ ID NO: 4, the HCDR2 comprises the amino acid sequence of SEQ ID NO: 5, the HCDR3 comprises the amino acid sequence of any one of SEQ ID NOs: 6, 11 or 12, the LCDR1 comprises the amino acid sequence of SEQ ID NO: 8, the LCDR2 comprises the amino acid sequence of SEQ ID NO: 9, and the LCDR3 comprises the amino acid sequence of SEQ ID NO: 10; wherein a cysteine is present in the VH domain at the amino acid corresponding to amino acid position 44 of SEQ ID NO: 94; and wherein a cysteine is present in the VL domain at the amino acid corresponding to amino acid position 103 of SEQ ID NO: 95.
|