| CPC C07K 16/1275 (2013.01) [A61K 9/0019 (2013.01); A61K 35/16 (2013.01); C07K 16/06 (2013.01); C07K 16/065 (2013.01); C07K 2317/10 (2013.01); C07K 2317/21 (2013.01)] | 15 Claims |
|
1. A method of treating an upper respiratory tract infection, the method comprising:
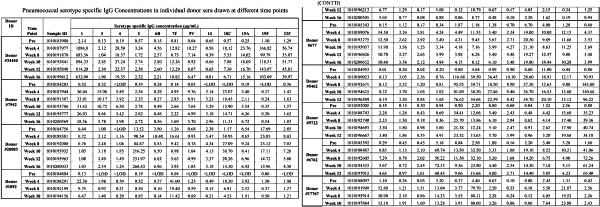
administering to a subject a therapeutically effective amount of an immunotherapeutic composition comprising immune globulin prepared from pooled plasma samples from healthy adult human plasma donors vaccinated with a primary and a secondary anti-pneumococcal vaccine, wherein the pooled plasma contains opsonophagocytic antibody titers specific for at least 7 or more S. pneumonia serotypes selected from the group consisting of S. pneumonia serotypes 1, 2, 3, 4, 5, 6A, 6B, 7A, 7B, 7C, 7D, 7E, 7F, 8, 9A-9V, 12, 14, 18C, 19A-19F, 23A-23F, and 25, wherein the opsonophagocytic antibody titers specific for the 7 or more S. pneumonia serotypes are each at least 3-fold higher than the opsonophagocytic antibody titers specific for the 7 or more S. pneumonia serotypes present in a control sample, wherein the control sample is immune globulin prepared from plasma pooled from 500 or more random human plasma donors that have not been vaccinated with an anti-pneumococcal vaccine.
|