| CPC C07D 513/14 (2013.01) [C07D 419/10 (2013.01); C07D 471/04 (2013.01); C07D 513/04 (2013.01); C07D 513/10 (2013.01); C07D 515/04 (2013.01); C07D 515/10 (2013.01); C07D 515/20 (2013.01); C07D 519/00 (2013.01)] | 17 Claims |
|
1. A compound of Formula (I) or a pharmaceutically acceptable salt thereof
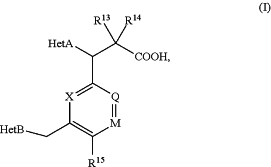 (I)
wherein
X is CH or N;
Q is CH or N;
M is CH;
wherein
when X is N, Q is CH;
when Q is N, X is CH;
R15 is CH3 or Cl;
R13 is H, F or C1-C4alkyl;
R14 is H, F or C1-C4alkyl;
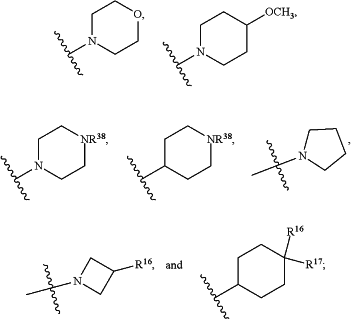
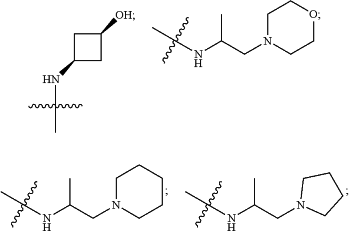
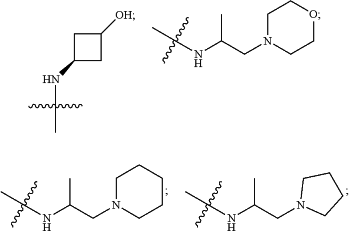
HetA is selected from the group consisting of
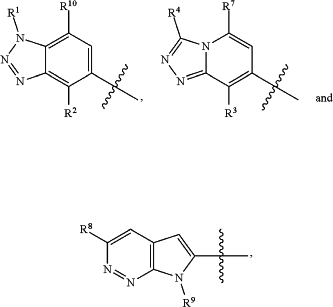 wherein
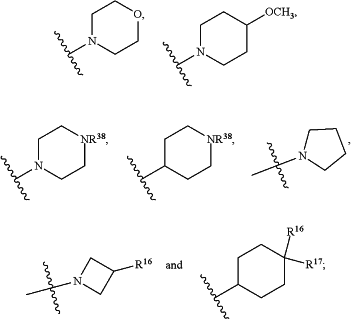
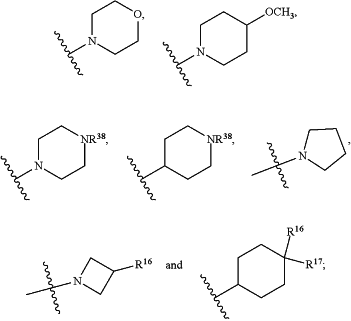
R1 is selected from the group consisting of C3-C4cycloalkyl, C1-C4alkyl and C1-C4alkyl monosubstituted with cyclopropyl or cyclobutyl;
R2 is selected from the group consisting of H, C1-C4alkyl and C1-C4perhaloalkyl;
R3 is H or C1-C4alkyl;
R4 is selected from the group consisting of C1-C4alkyl, C1-C4perhaloalkyl and C3-C4cycloalkyl;
R7 is H or C1-C4alkyl;
R8 is C1-C4alkyl;
R9 is C1-C4alkyl;
R10 is selected from the group consisting of H, —OC3-C4cycloalkyl and —OC1-C4perhaloalkyl;
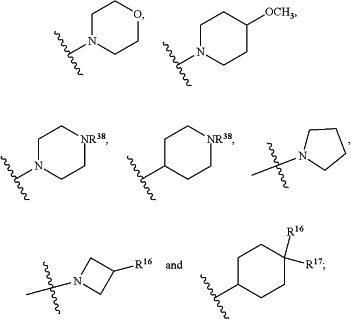
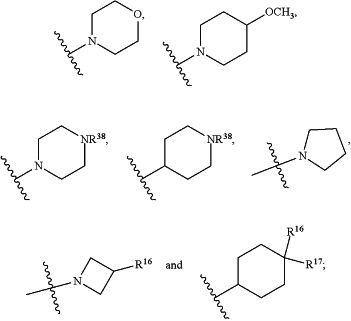
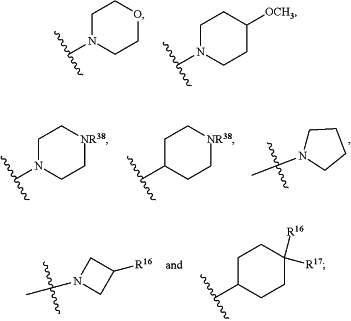
HetB is selected from the group consisting of
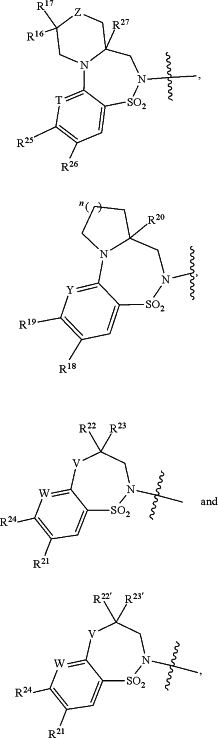 wherein
Z is selected from the group consisting of O, CH2, NH and N(CH3);
T is CH or N;
Y is CH or N;
W is CH or N;
V is O or N(CH3);
R16 is H or F;
R17 is H or F;
n is 0, 1 or 2;
R18 is selected from the group consisting of H, —CN, halo, C(O)NH2, C1-C4alkyl and C1-C4perhaloalkyl;
R19 is selected from the group consisting of H; CN; halo; C(O)NH2;
N(R38)C1-C6alkyl; C1-C4alkyl; C1-C4perhaloalkyl;
 OC1-C6alkyl;
OC1-C6alkyl substituted with one or two substituents selected from the group consisting of —OH, —OCH3, —O(CH2)3OH, —N(R36)R37, C1-C4alkyl,
 —N(R38)C1-C6alkyl substituted with one or two substituents selected from the group consisting of OH, —OCH3, —N(R36)R37, C1-C4alkyl,
 and
C1-C4alkyl monosubstituted with a substituent selected from the group consisting of —C(O)NHCH2CH2OH, —C(O)NHCH2CH2OCH2CH2NH2, C(O)NH2 and OH;
R20 is H or C1-C4alkyl;
R21 is selected from the group consisting of H, —CN, halo, C1-C4alkyl and C1-C4perhaloalkyl;
R22 and R23 are taken together with the carbon to which they are attached to form
(a) the moiety
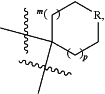 wherein R is selected from the group consisting of CH2, NR38 and O, m is 0 or 1, and p is 0 or 1; or
(b) the moiety
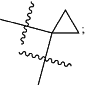 R22′ is selected from the group consisting of H, C1-C4alkyl and C3-C4cycloalkyl, and
R23′ is selected from the group consisting of H, C1-C4alkyl and C3-C4cycloalkyl;
R24 is selected from the group consisting of H; CN; halo; C(O)NH2;
C(O)(NH)C3-C4cycloalkyl; N(R38)C1-C6alkyl; C1-C4alkyl; C1-C4perhaloalkyl;
 OC1-C6alkyl;
OC1-C6alkyl substituted with one or two substituents selected from the group consisting of —OH, —OCH3, —O(CH2)3OH, —N(R36)R37, C1-C4alkyl;
 N(R38)C1-C6alkyl substituted with one or two substituents selected from the group consisting of OH, —OCH3, —N(R36)R37, C1-C4alkyl,
 and
C1-C4alkyl monosubstituted with a substituent selected from the group consisting of —C(O)NHCH2CH2OH, —C(O)NHCH2CH2OCH2CH2NH2, C(O)NH2 and OH;
R25 is selected from the group consisting of H; CN; halo; C(O)NH2;
N(R38)C1-C6alkyl; C1-C4alkyl; C1-C4perhaloalkyl;
 OC1-C6alkyl;
OC1-C6alkyl substituted with one or two substituents selected from the group consisting of —OH, —OCH3, —O(CH2)3OH, —N(R36)R37, C1-C4alkyl,
 —N(R38)C1-C6alkyl substituted with one or two substituents selected from the group consisting of —OH, —OCH3, —N(R36)R37, C1-C4alkyl,
 and
C1-C4alkyl monosubstituted with a substituent selected from the group consisting of —C(O)NHCH2CH2OH, —C(O)NHCH2CH2OCH2CH2NH2, C(O)NH2 and OH;
R26 is selected from the group consisting of H, —CN, halo, C1-C4alkyl and C1-C4perhaloalkyl;
R27 is H or C1-C4alkyl;
R36 and R37 are independently selected from the group consisting of H and C1-C4alkyl;
R38 is H or C1-C4alkyl;
provided that when HetA is
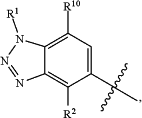 then HetB is not
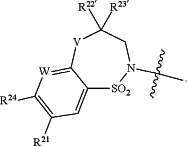 |
|
17. A pharmaceutical composition comprising an effective amount of a compound as claimed in claim 1 and at least one pharmaceutical acceptable excipient.
|