| CPC C07D 231/20 (2013.01) [A61K 31/415 (2013.01); A61K 31/4155 (2013.01); A61K 31/4178 (2013.01); A61K 31/422 (2013.01); A61K 31/427 (2013.01); A61K 31/437 (2013.01); A61K 31/4439 (2013.01); A61K 31/454 (2013.01); A61K 31/496 (2013.01); A61K 31/501 (2013.01); A61K 31/506 (2013.01); A61K 31/519 (2013.01); A61K 31/5355 (2013.01); A61K 45/06 (2013.01); C07D 231/38 (2013.01); C07D 401/04 (2013.01); C07D 403/04 (2013.01); C07D 403/06 (2013.01); C07D 409/04 (2013.01); C07D 413/04 (2013.01); C07D 417/04 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 495/04 (2013.01); C07D 495/14 (2013.01)] | 19 Claims |
|
1. A compound or salt of formula (Ib):
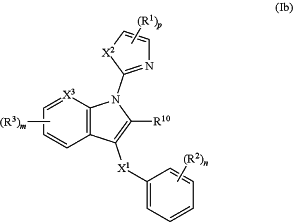 wherein
R1 is independently chosen from halo, —CO2R4, —C(O)NR5R6, —(C1-C8hydrocarbyl), —C(O)NHOH, —C(O)OCR5R6OC(O)OR4, —(C0-C4hydrocarbyl)((mono- or bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S), —C(O)O—(C0-C4hydrocarbyl)(mono- or bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S), —P(O)(OH)2, —B(OR13)(OR14), —SO2(OH), —C(O)NHS(O)2Me and —SO2NR5R6, each of which R1 except halo is substituted or unsubstituted;
R2 is independently chosen from hydroxyl, halo, —CN, —NO2, C1-C8hydrocarbyl, —O(C1-C8hydrocarbyl), —(C0-C4hydrocarbyl)C3-C8cycloalkyl, —O(C0-C4hydrocarbyl)C3-C8 cycloalkyl, —(C0-C4hydrocarbyl)C3-C8cycloalkenyl, —O(C0-C4hydrocarbyl)C3-C8cycloalkenyl, —O(C0-C4hydrocarbyl)C6-C12aryl, —(C0-C4hydrocarbyl)C6-C12aryl, —O(C0-C4hydrocarbyl)(mono- and bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S), —(C0-C4hydrocarbyl)(mono- and bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S), —C(O)R4, —CO2R4, —C(O)NR5R6, —NR5C(O)R4, —(CH2)qNR5(SO2)R4, —(CH2)qNR5C(O)R4, —(CH2)qNR7C(O)NR5R6, —(CH2)qNR5R6, —(CH2)qSO2NR5R6, —(CH2)qSO2R4, —(CH2)qaryl, —(CH2)qheteroaryl, or —(CH2)qheterocycloalkyl, each of which C1-C8hydrocarbyl, —O(C1-C8hydrocarbyl), —(C0-C4hydrocarbyl)C3-C8 cycloalkyl, —O(C0-C4hydrocarbyl)C3-C8 cycloalkyl, —(C0-C4hydrocarbyl)C3-C8 cycloalkenyl, —O(C0-C4hydrocarbyl)C3-C8 cycloalkenyl, —O(C0-C4hydrocarbyl)C6-C12aryl, —(C0-C4hydrocarbyl)C6-C12aryl, —O(C0-C4hydrocarbyl)(mono- and bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S), —(C0-C4hydrocarbyl)(mono- and bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S) is substituted or unsubstituted;
R3 is independently chosen from hydroxyl, halo, —CN, —NO2, —SF5, C1-C8hydrocarbyl, —O(C1-C8hydrocarbyl), —(C0-C4hydrocarbyl)C3-C8 cycloalkyl, —O(C0-C4hydrocarbyl)C3-C8 cycloalkyl, —(C0-C4hydrocarbyl)C3-C8 cycloalkenyl, —O(C0-C4hydrocarbyl)C3-C8 cycloalkenyl, —O(C0-C4hydrocarbyl)C6-C12aryl, —(C0-C4hydrocarbyl)C6-C12aryl, —O(C0-C4hydrocarbyl)(mono- and bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S), —(C0-C4hydrocarbyl)(mono- and bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S), —C(O)R4, —CO2R4, —C(O)NR5R6, —NR5C(O)R4, —(CH2)qNR5(SO2)R4, —(CH2)qNR5C(O)R4, —(CH2)qNR7C(O)NR5R6, —(CH2)qNR5R6, —(CH2)qSO2NR5R6, —(CH2)qSO2R4, each of which C1-C8hydrocarbyl, —O(C1-C8hydrocarbyl), —(C0-C4hydrocarbyl)C3-C8 cycloalkyl, —O(C0-C4hydrocarbyl)C3-C8 cycloalkyl, —(C0-C4hydrocarbyl)C3-C8 cycloalkenyl, —O(C0-C4hydrocarbyl)C3-C8 cycloalkenyl, —O(C0-C4hydrocarbyl)C6-C12aryl, —(C0-C4hydrocarbyl)C6-C12aryl, —O(C0-C4hydrocarbyl)(mono- and bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S), —(C0-C4hydrocarbyl)(mono- and bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S) is substituted or unsubstituted; or
each R4, R5, R6, R7, R8, and R9 is the same or different and each is hydrogen, C1-C8 alkyl, C2-C8 alkenyl, C3-C6 cycloalkyl, aryl, heteroaryl, or heterocycloalkyl, each of which C1-C8 alkyl, C2-C8 alkenyl, C3-C6 cycloalkyl, aryl, heteroaryl, or heterocycloalkyl is substituted or unsubstituted;
R10 is hydrogen, halo, —CN, —NO2, —CO2R4, —C(O)NR5R6, —NR5(SO2)R4, —NR5C(O)R4, —NR7C(O)NR5R6, —NR5R6, —SO2NR5R6, —SO2R4, C1-C8hydrocarbyl, —O(C1-C8hydrocarbyl), —(C0-C4hydrocarbyl)C3-C8 cycloalkyl, —O(C0-C4hydrocarbyl)C3-C8 cycloalkyl, —(C0-C4hydrocarbyl)C3-C8 cycloalkenyl, —O(C0-C4hydrocarbyl)C3-C8 cycloalkenyl, —O(C0-C4hydrocarbyl)C6-C12aryl, —(C0-C4hydrocarbyl)C6-C12aryl, —O(C0-C4hydrocarbyl)(mono- and bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S), —(C0-C4hydrocarbyl)(mono- and bicyclic heterocycle having 1 to 4 heteroatoms independently chosen from N, O, and S), each of which R10 except hydrogen, halo, —CN, and —NO2 is substituted or unsubstituted;
each R13 and R14 is the same or different and each is hydrogen, C1-C8 alkyl, C2-C8 alkenyl, C3-C6 cycloalkyl, C6-C12 aryl, wherein R13 and R14 are optionally connected to each other to form a ring;
X1 is a bond, —CR8R9—, —NR5—, —CR8NR5—, —NR5CR8—, —NR5C(O)—, —O—, —CO—, —SO—, —SO2—, or —S—;
X2 is —NR5—, —O—, or —SO2—, or —S—;
X3 is CH or N;
n is an integer from 1-5;
m is an integer from 1-5;
o is 0 or an integer from 1-5; and
p is 1 or 2.
|